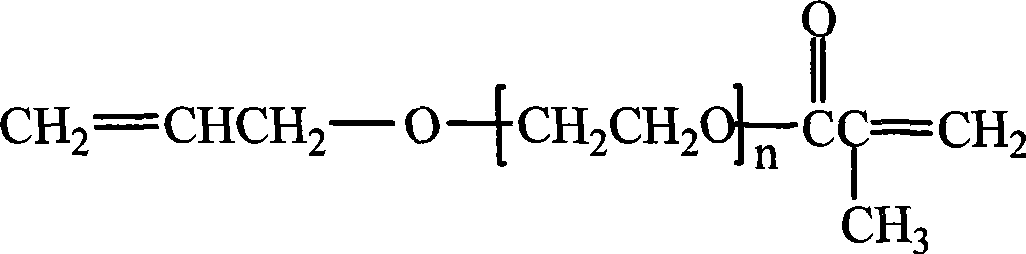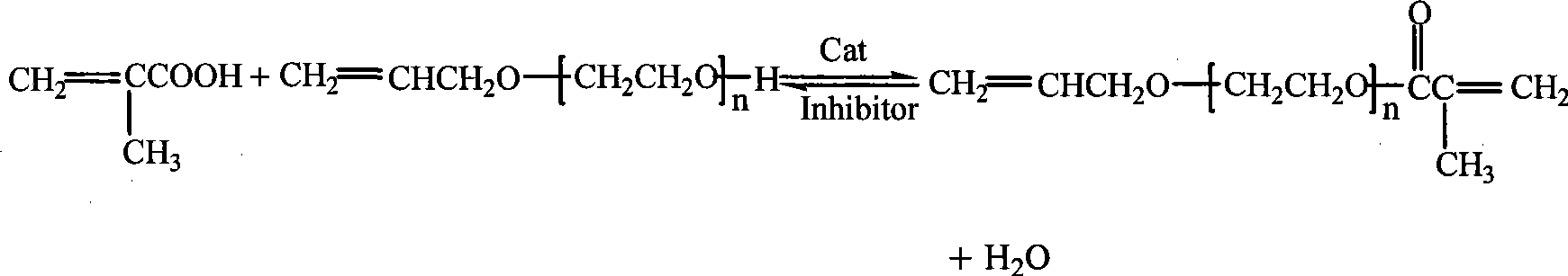Method for synthesizing allyl alcohol polyethenoxy ether metacrylic acid ester
A technology of allyl alcohol polyoxyethylene ether methacrylate and allyl alcohol polyoxyethylene ether, which is applied in the field of synthesis of ester compounds in organic chemistry, to achieve the effects of shortened reaction time, mild conditions, and safe and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: Add allyl alcohol polyoxyethylene ether 174.2g (polymerization degree is 5), methacrylic acid 72.6g, gac solid 13.5g of phosphotungstic acid, 0.25g of hydroquinone as a polymerization inhibitor, 201g of water-carrying agent (volume ratio of toluene:cyclohexane=35:65), the temperature is raised to 100-105°C under stirring, and nitrogen gas is introduced. React for 5.5 hours, when the water-carrying agent does not react and produce water, it is the end of the reaction. Turn off the nitrogen, cool down to 60-70°C, filter and remove the catalyst phosphotungstic acid (recovered for reuse), and then add 0.49g of diatomaceous earth to the filtrate , stirred for about 30 minutes, filtered, and the solvent was distilled off under reduced pressure to obtain allyl alcohol polyoxyethylene ether methacrylate, the acid value was less than 0.5mgKOH / g, and the esterification rate was 98.5%.
Embodiment 2
[0017] Embodiment 2: Add allyl alcohol polyoxyethylene ether 177.6g (polymerization degree is 9), methacrylic acid 48.2g, gac solid 18.0g of phosphotungstic acid, 0.18g of polymerization inhibitor phenothiazine, 225.8g of water-carrying agent (volume ratio of toluene: cyclohexane = 45:55), the temperature is raised to 110-115°C under stirring, and nitrogen gas is introduced. React for 6.0 hours, when the water-carrying agent does not react and generate water, it is the end of the reaction. Turn off the nitrogen, lower the temperature to 60-70°C, filter and remove the catalyst phosphotungstic acid (recovered for reuse), and then add 0.68g of diatomaceous earth to the filtrate , stirred for about 30 minutes, filtered, and the solvent was distilled off under reduced pressure to obtain allyl alcohol polyoxyethylene ether methacrylate, the acid value was less than 0.5mgKOH / g, and the esterification rate was 98.0%.
[0018] In the above-mentioned embodiment, reaction equation is:
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com