Method for photocatalytic synthesis of 1,3,5-trisubstituted-2-pyrazole derivative
A technology of pyrazole derivatives and three substitutions, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
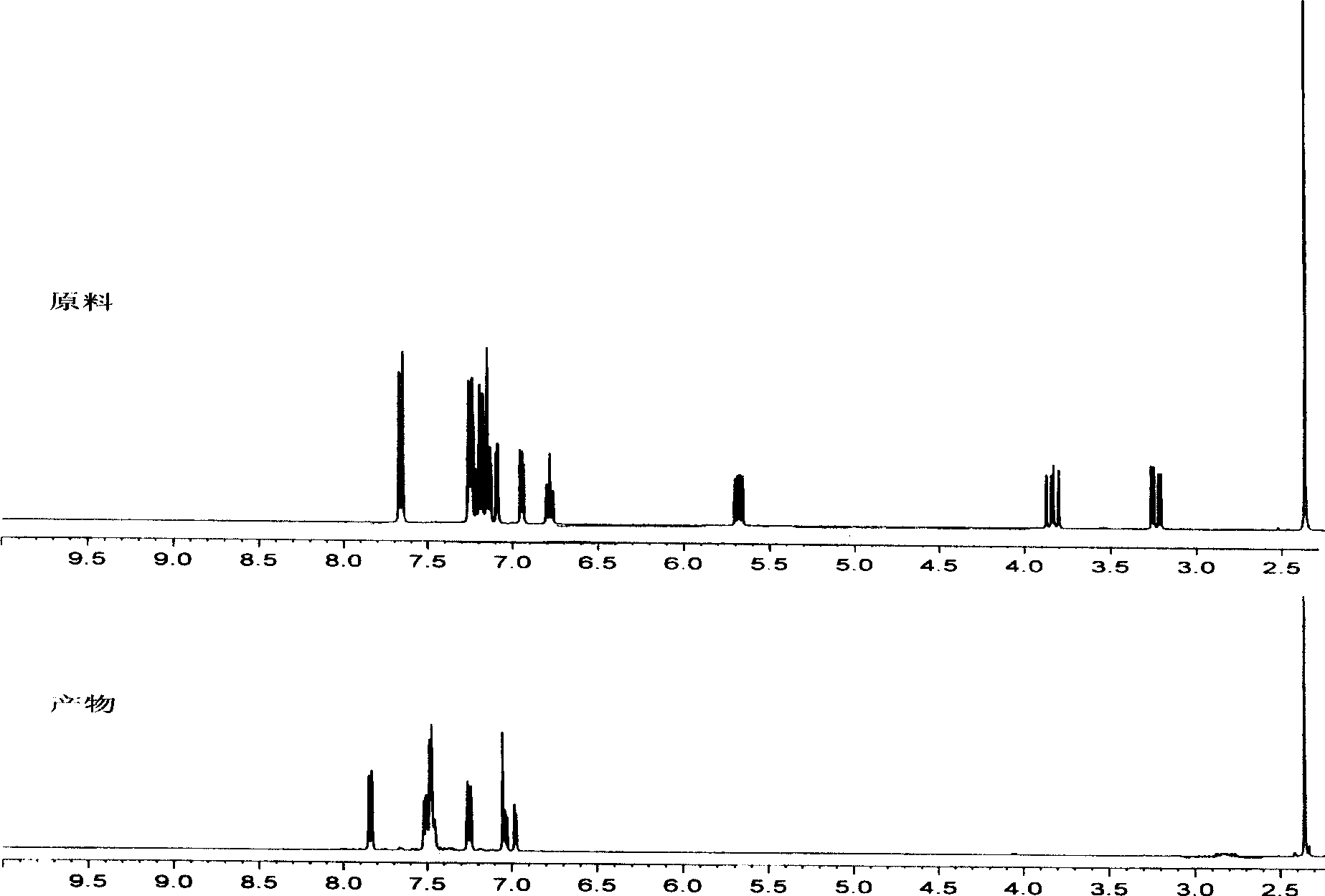
[0047] Under argon protection, irradiate 1,3,5-triphenyl-2-pyrazoline with a 500W high-pressure mercury lamp (concentration is 10 -2 mol / l in 40ml CH 3 CN solution) with a catalytic amount of 2,2':6',2"-terpyridine divalent platinum complex (wherein R 1 for C 6 h 4 OCH 3 -4, R 2 is C≡CC 6 h 4 C≡CC 6 h 5 , R 3 , R 4 independent for H, R 5 for ClO 4 - , in 40ml CH 3 The concentration in CN solution is 10 -5 mol / l) acetonitrile solution, using a 480nm glass filter, monitor the reaction with thin layer chromatography, proton nuclear magnetic spectrum or fluorescence. After the reaction, evaporate the solvent, add ethyl acetate to extract, filter, spin the filtrate to obtain the product, and recover the insoluble catalyst. The product was identified as 1,3,5-triphenyl-2-pyrazole by H NMR and mass spectrometry.
Embodiment 2
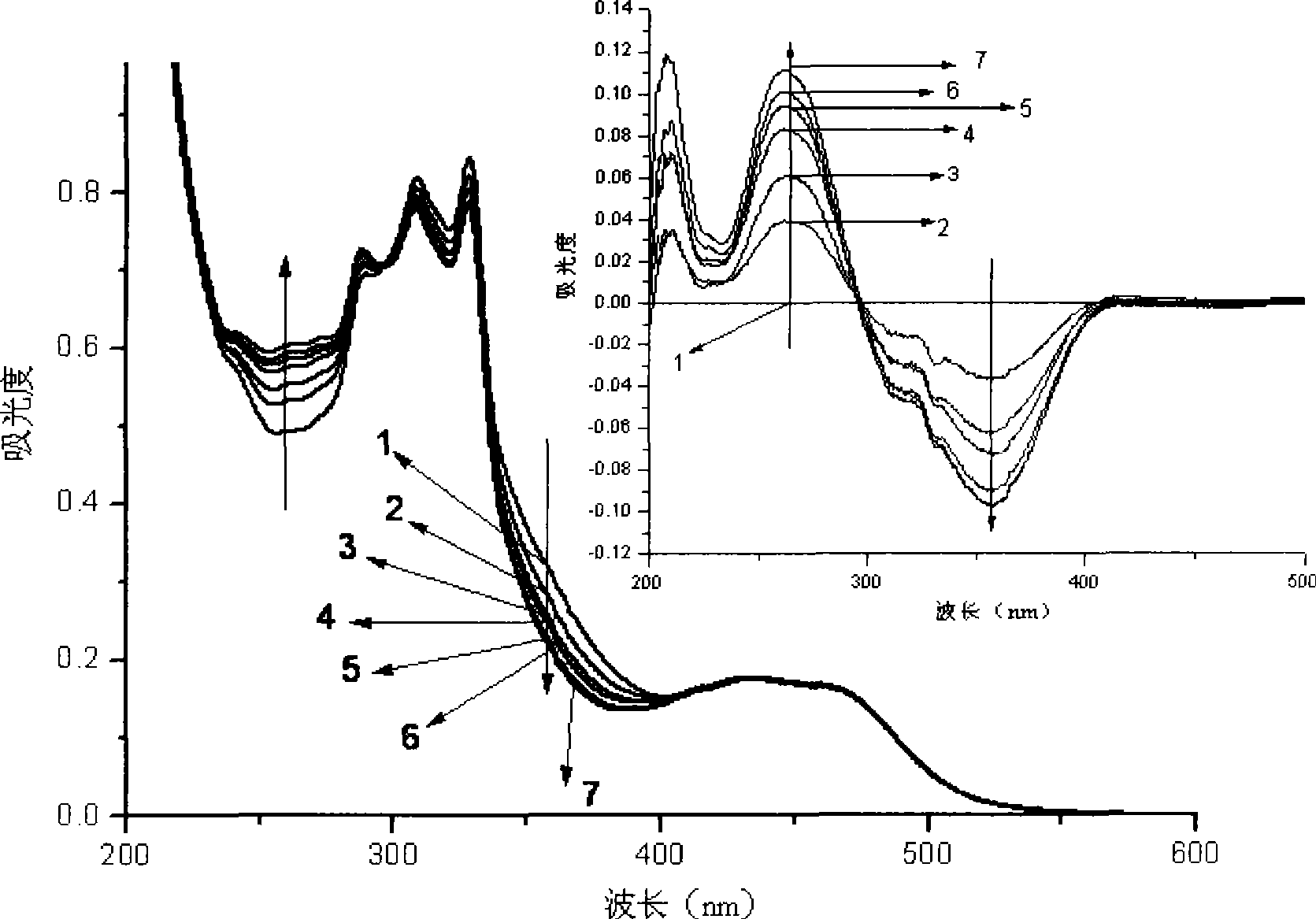
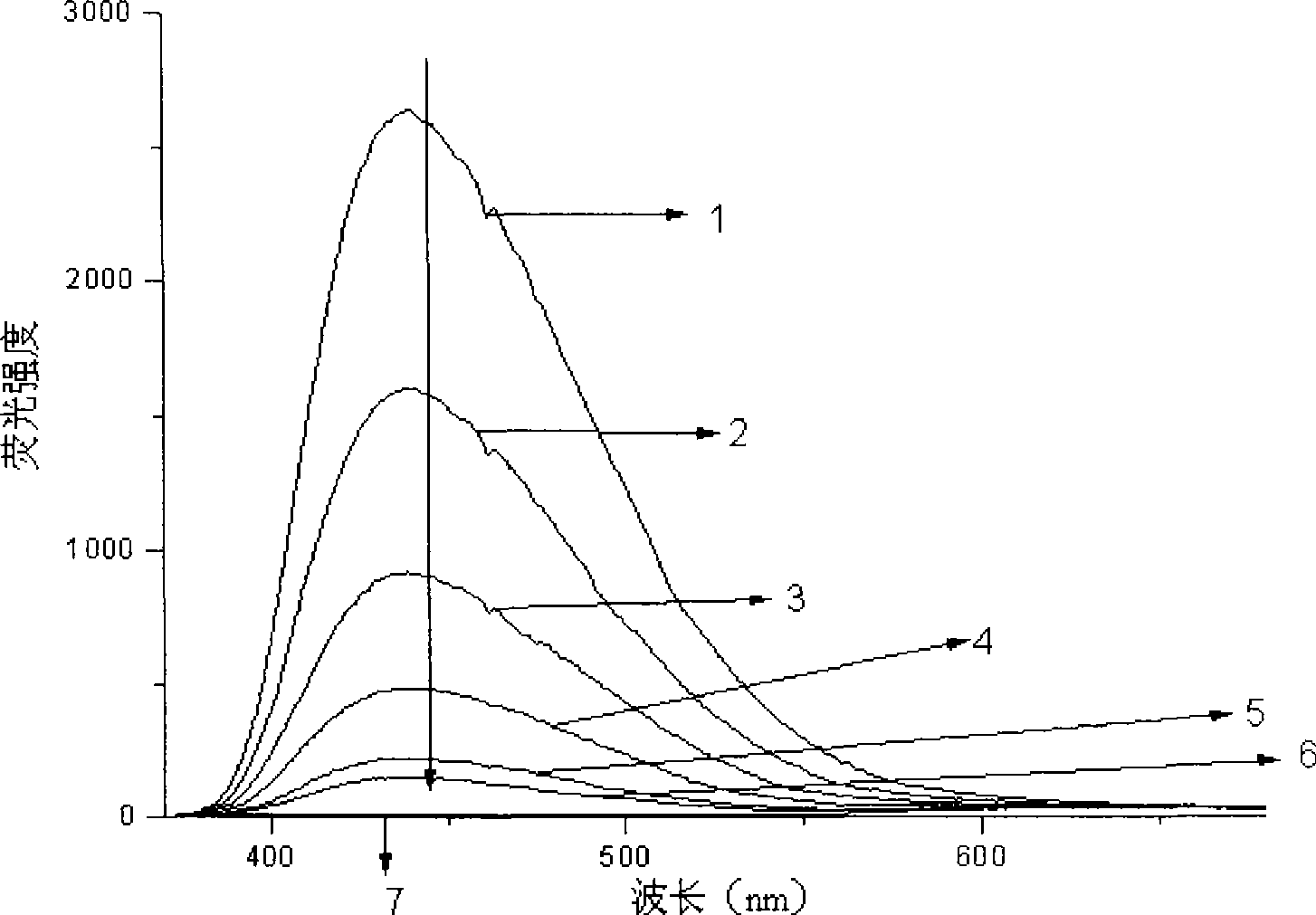
[0049] Under argon protection, irradiate 1,3-diphenyl-5-p-methylphenyl-2-pyrazoline (concentration is 10-2mol / l, in 40ml methanol solution) and catalytic amount with 500W high-pressure mercury lamp The 2,2':6',2"-terpyridine divalent platinum complex (wherein R 1 for C 6 h 4 OCH 3 -4, R 2 is C≡CC 6 h 4 C≡CC 6 h 5 , R 3 , R 4 independent for H, R 5 for ClO 4 - , the concentration in 40ml methanol solution is 10 -5 mol / l) in methanol solution, irradiated with light with a wavelength of 450nm, and monitored the reaction with thin layer chromatography, nuclear magnetic hydrogen spectrum or fluorescence. After the reaction, evaporate the solvent, add ethyl acetate to extract, filter, spin the filtrate to obtain the product, and recover the insoluble catalyst. The product was identified as 1,3-diphenyl-5-p-methylphenyl-2-pyrazole by H NMR and mass spectrometry. The absorption spectrum of the reaction process is as figure 1 shown.
Embodiment 3
[0051] Under argon protection, compound 1,3-diphenyl-5-p-methylphenyl-2-pyrazoline was irradiated with a 500W high-pressure mercury lamp (concentration was 10 -2 mol / l, in 40ml methanol solution) with a catalytic amount of 2,2':6',2"-terpyridine divalent platinum complex (wherein R 1 for C 6 h 4 CH 3 -4, R 2 for Cl, R 3 , R 4 independent for H, R 5 for Cl - , the concentration in 40ml methanol solution is 10 -5 mol / l) of methanol solution, irradiated with light with a wavelength of 410nm, and monitored the reaction with thin layer chromatography, nuclear magnetic hydrogen spectrum or fluorescence. After the reaction, evaporate the solvent, add ethyl acetate to extract, filter, spin the filtrate to obtain the product, and recover the insoluble catalyst. The product was identified as 1,3-diphenyl-5-p-methylphenyl-2-pyrazole by H NMR and mass spectrometry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com