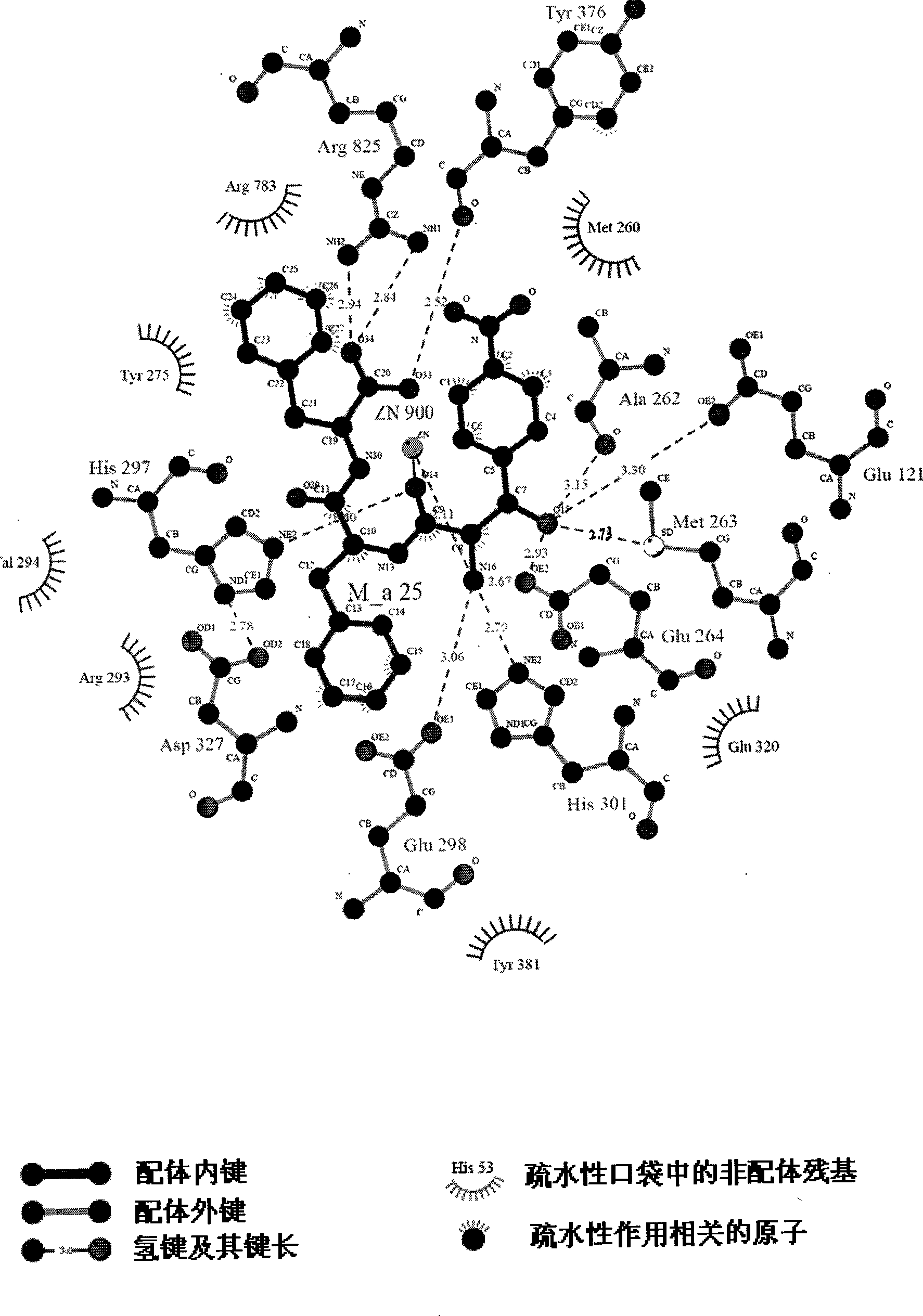
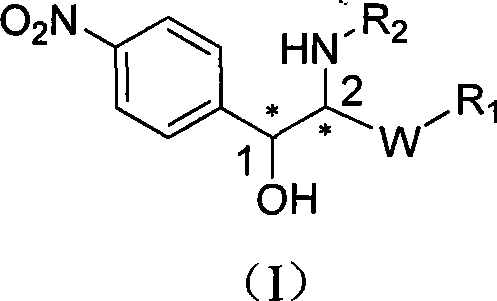
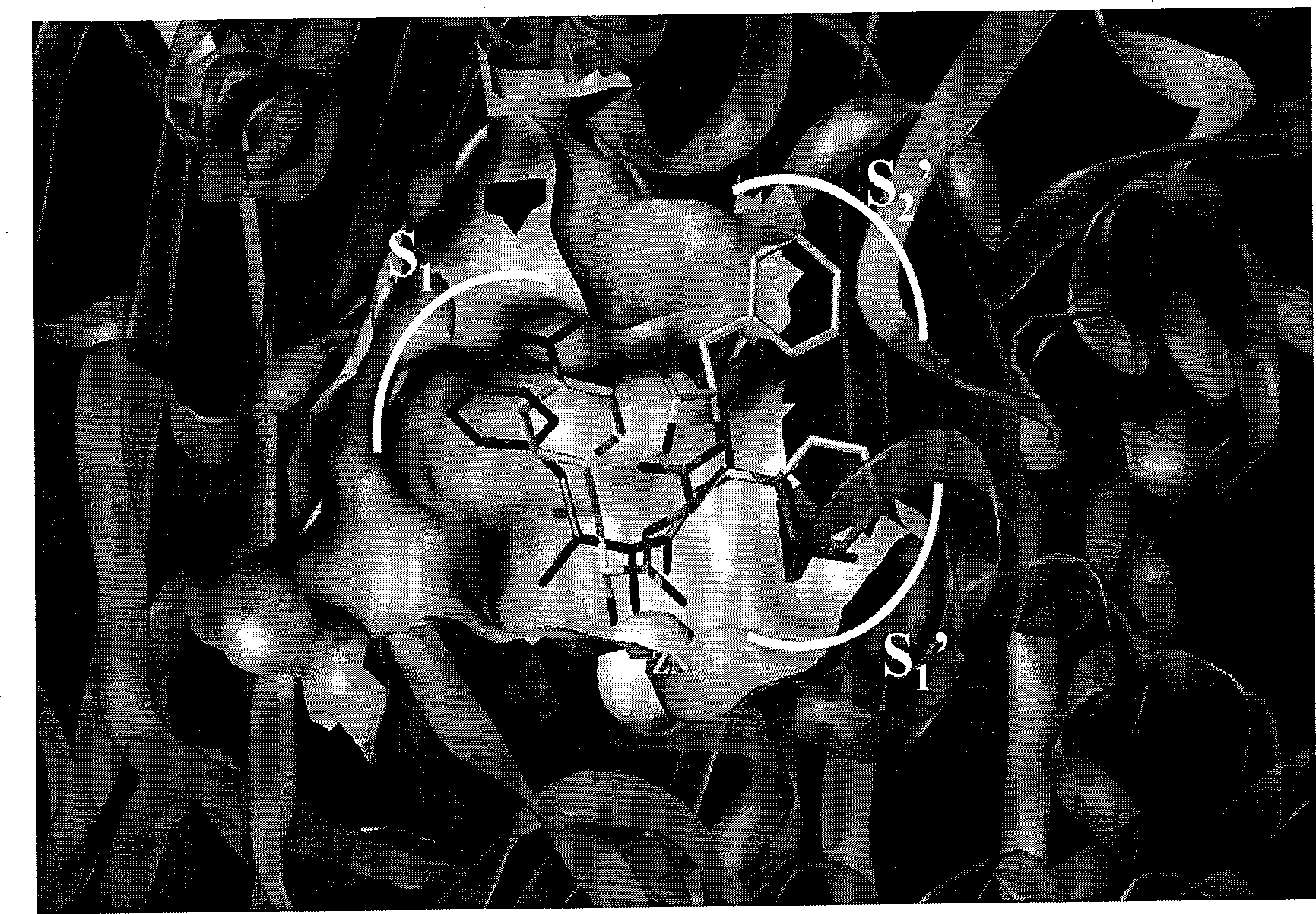2-amino-1-(4-nitro phenyl)-1-ethanol metalloid protease inhibitor, and preparation and use thereof
A technology of nitrophenyl and amino, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0106] The terms and definitions used in this document have the following meanings:
[0107] "Heteroalkyl" means a saturated or unsaturated chain comprising carbon atoms and at least one heteroatom, none of which are adjacent. The heteroalkyl group contains 2-15 atoms (carbon atoms), preferably 2-10 atoms. Heteroalkyl groups can be straight or branched, substituted or unsubstituted.
[0108] "Aryl" means an aromatic carbocyclic group. Preferred aromatic rings contain 6-10 carbon atoms.
[0109] "Halo", or "halogen" includes fluorine, chlorine, bromine or iodine, preferably fluorine and chlorine.
[0110] "Cycloalkyl" is a substituted or unsubstituted, saturated or unsaturated cyclic group containing carbon atoms and / or one or more heteroatoms. The ring may be a monocyclic or fused, bridged or spiro ring system. Monocyclic rings generally contain 3-9 atoms, preferably 4-7 atoms, and polycyclic rings contain 7-17 atoms, preferably 7-13 atoms.
[0111] "Heteroaryl" is an ar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com