QSOX, mutant protein thereof, as well as preparation and use
A mutant and protein technology, applied in the field of QSOX and its mutant protein and preparation, can solve the problem of undiscovered yeast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Example 1 Construction and expression of DPICZaA-Q6604 vector system
[0099] Total RNA was extracted from fibroblasts using Trizol reagent. The extracted total RNA was used as a template, reverse-transcribed into cDNA by RT-PCR, using primers P1: 5'-GTActc gag aaa agaATGAGGAGGTGCAACAGCGGCTCCG---3' and P2: 5'-CAGgcg gcc gcTCAAATAAGCTCAGGTCCCTCAG-3', amplified by PCR The QSOX gene coding sequence was obtained, and the QSOX gene fragment was loaded into the pGEM-T vector. Then it was digested with Xhol I and Not I, and subcloned into pPICZaA to obtain pPICZaA-Q6604. Sequencing results and analysis such as Figure 2A and Figure 2B shown.
[0100] After the correctly constructed pPICZaA-Q6604 was linearized with SalI, the yeast was electrotransformed according to the method in "Molecular Cloning: Experiment Guide". The obtained recombinant yeast was screened for copy number by Zeocin resistance, identified correctly by PCR, and induced expression with methanol as ...
Embodiment 2
[0101] Example 2 Construction and expression of pET28a-Q6604-70m vector system
[0102] See figure 1As shown, using the QSOX gene loaded on the pGEM-T vector as a template, using primers Pm70-1-5'GGAGTTCTTCGCCTCCTGGGCCGGCCACGCCATCGCCTTCGCCC-3 and Pm70-2-5GGGCGAAGGCGATGGCGTGGCCGGCCCAGGAGGCGAAGAACTCC-3 to mutate the Cys at positions 70 and 73 of the QSOX protein to Ala, and obtain The mutated gene was double digested with NdeI and Not I, and then directional cloned into pET28a. After the obtained recombinant vector was identified correctly, it was transformed into Escherichia coli, and the recombinant strain was induced in LB liquid medium with a final concentration of 0.3mM IPTG at 18°C for 24 hours for small expression, and QSOX enzyme binding factor FAD10μM was added during the induction, and the induction ended At 6000rmp, the supernatant was centrifuged to collect the precipitate. Recombinant protein QSOX was expressed in Escherichia coli to obtain soluble protein....
Embodiment 3
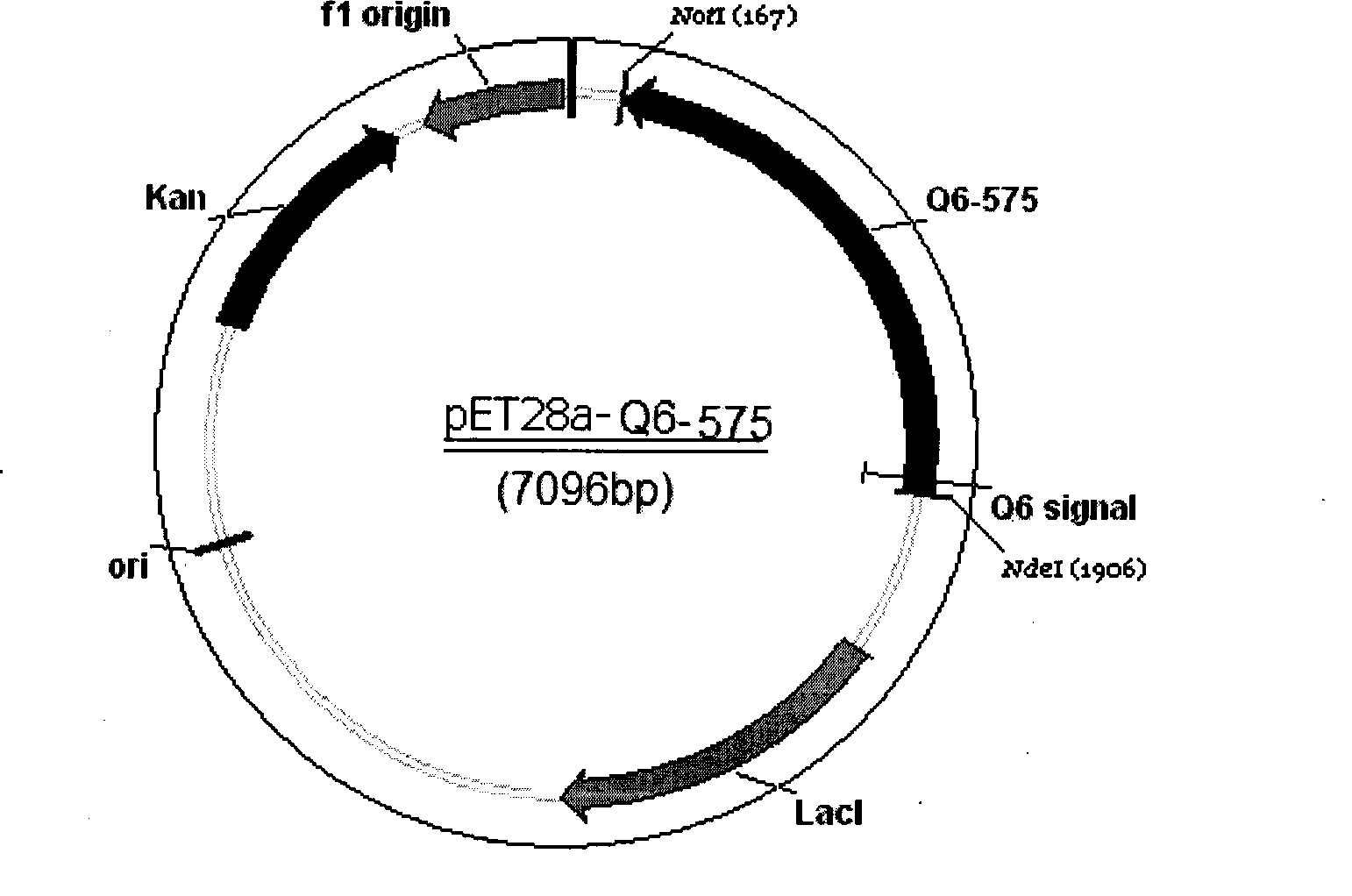
[0103] Example 3 Construction and expression of pTYB12-Q6575 vector system
[0104] Using the QSOX gene loaded into the pGEM-T vector as a template, PCR amplification was performed using primers P30N5'tatcatatgGCCCCGCGGTCGGCGCTCTATTCGCCTT-3' and P2: 5'-CAGgcg gcc gcTCAAATAAGCTCAGGTCCCTCAG-3', and the amplified product was QSOX without the signal peptide The coding sequence of the gene is called Q6575, and the QSOX gene fragment is double digested with NdeI and Not I, and then directionally cloned into pTYB12. After the obtained recombinant vector was identified correctly, it was transformed into Escherichia coli, and the recombinant strain was induced in LB liquid medium with a final concentration of 0.3mM IPTG at 18°C for 24 hours for small expression, and QSOX enzyme binding factor FAD10μM was added during the induction, and the induction ended At 6000rmp, the supernatant was centrifuged to collect the precipitate. Recombinant protein QSOX was expressed in Escherichia...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com