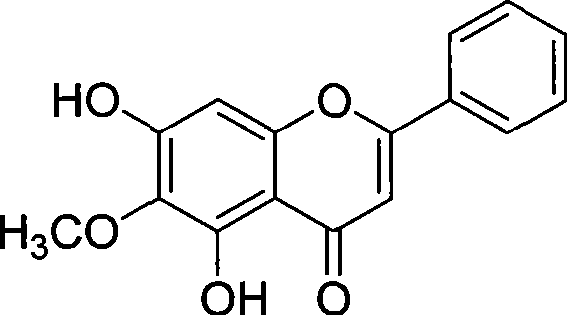
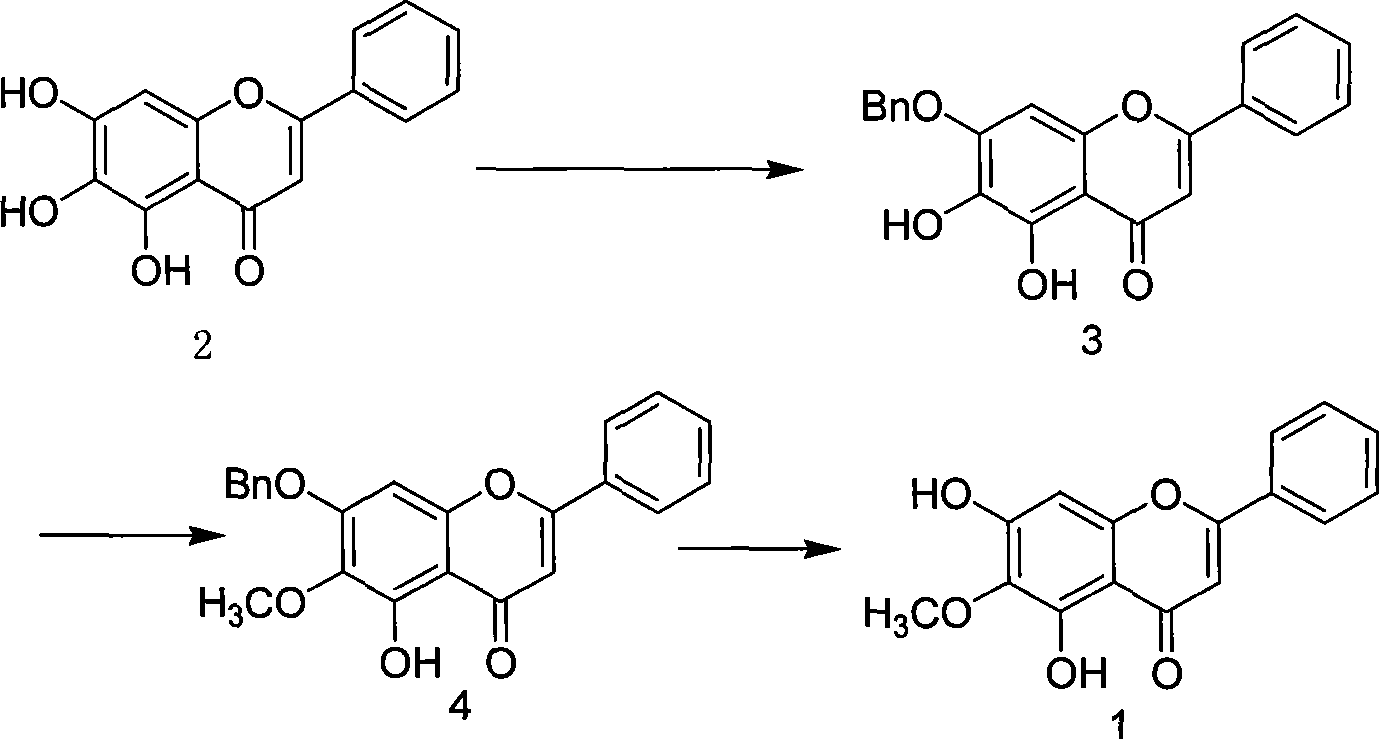
Synthesis of oroxylin
A synthesis method, the technology of melaleuca, applied in the directions of drug combination, digestive system, antipyretics, etc., can solve the problem of high cost, and achieve the effects of simple steps, high product purity, and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 7-benzyl-5,6-dihydroxyflavone
[0031] Add 16g of baicalein and 1600mL of acetone in a 2000ml reaction flask equipped with a mechanical stirrer, a thermometer, and a nitrogen inlet tube, and feed N 2 Replace the air, keep the nitrogen flowing, heat to 32°C to completely dissolve the baicalein, add K 2 CO 3 59g, 10mL of benzyl bromide, reacted for 48h, cooled, concentrated to dryness under reduced pressure, added 200ml of water, stirred, filtered, and dried to obtain 15.5g of yellow solid, yield 92%, Mp: 195°C-196°C. 10% FeCl 3 Ethanol solution is dark green
[0032] 1 H-NMR (DMSO-d 6 , 300Hz): δ 5.30 (2H, s, CH 2 Ph), 7.01 (1H, s, 3H), 7.12 (1H, S, 8H) 7.33-7.65 (10H, m, ArH), 8.08-8.10 (2H, d, ArH), 8.83 (1H, s, 6- OH), 12.55 (1H, s, 5-OH) MS (EI, m / z) 360, 269, 241, 139, 91
Embodiment 2
[0034] Preparation of 7-benzyloxy-5,6-dihydroxyflavone
[0035] Add baicalein 16g (59mmol) and ethanol 1500mL in the 2000ml reaction bottle that mechanical stirrer, thermometer are housed, nitrogen inlet pipe, pass into N 2 Replace the air, keep the nitrogen flowing, heat to 40°C to completely dissolve the baicalein, add Na 2 CO 3 55g, 15mL of benzyl bromide, reacted for 24h, cooled, concentrated to dryness under reduced pressure, added 200ml of water, stirred, filtered, and dried to obtain 14.0g of yellow solid, yield 77%, Mp: 195°C-196°C. 10% FeCl 3 The ethanol solution is dark green.
Embodiment 3
[0037] Preparation of 7-benzyloxy-5,6-dihydroxyflavone
[0038] Add baicalein 16g (59mmol) in the 2000ml reaction bottle that mechanical stirrer, thermometer are housed, nitrogen inlet tube, acetone 1500mL, pass into N 2 Replace the air, heat to 40°C to dissolve the baicalein completely, add KHCO 3 59g, 15mL of benzyl bromide, reacted for 24h, cooled, concentrated to dryness under reduced pressure, added 200ml of water, stirred, filtered, and dried to obtain 13.0g of yellow solid, yield 82%, Mp: 195°C-196°C. 10% FeCl 3 The ethanol solution is dark green.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com