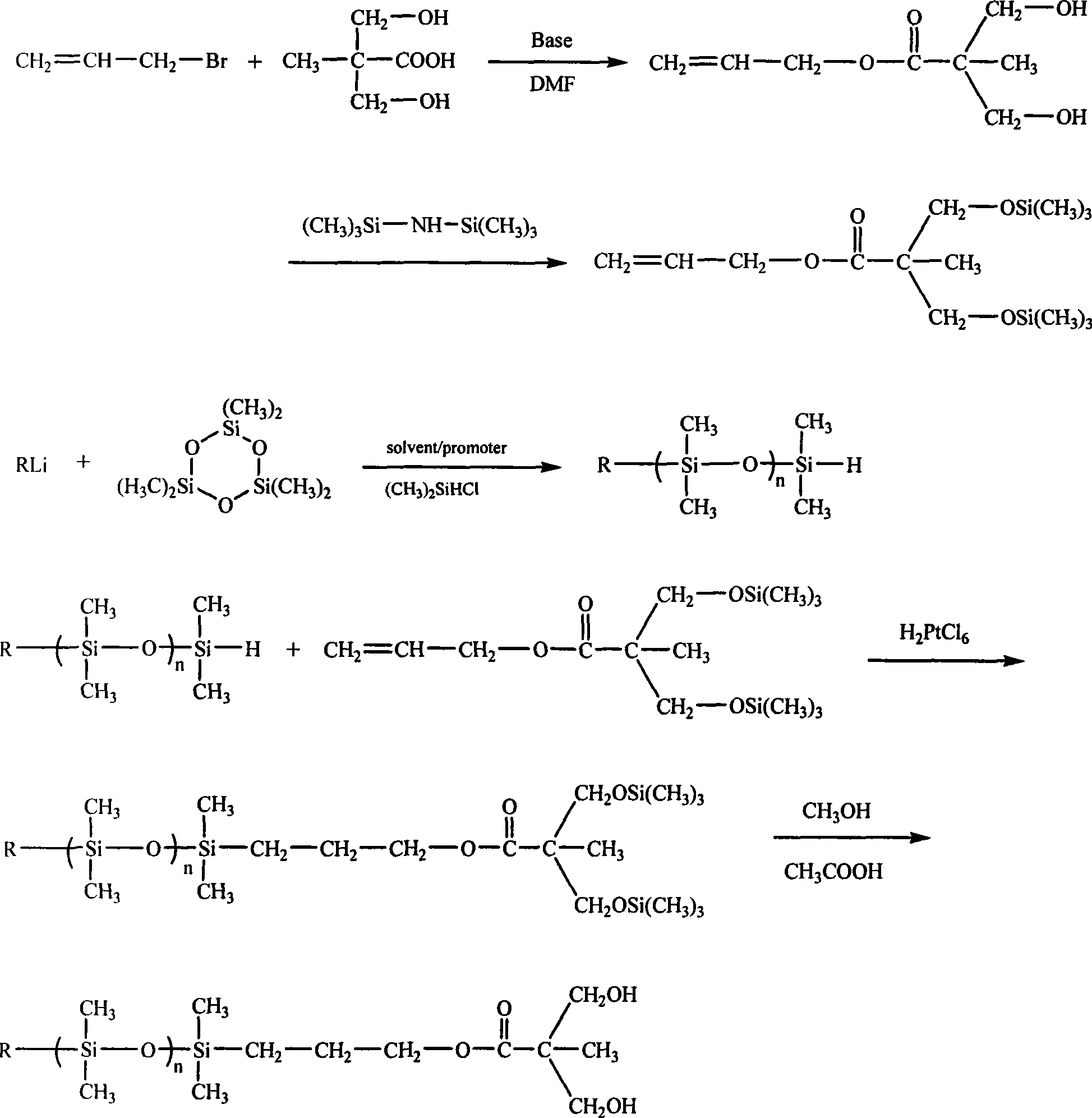Synthesis of polysiloxane containing bishydroxymethyl at single end
A polysiloxane and bismethylol technology, which is applied in the field of polysiloxane synthesis, can solve the problems such as difficult to obtain unsaturated compounds and harsh reaction conditions for unsaturated compounds, and achieve easy separation and purification, mild conditions, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Esterification reaction (molar ratio 2,2-dimethylolpropionic acid: allyl bromide: potassium carbonate = 1: 1.2: 0.5)
[0025] In a 250mL three-necked flask, add 13.37g of 2,2-dimethylolpropionic acid, 7.00g of potassium carbonate and 100mL of N,N-dimethylformamide. The temperature of the reaction system was raised to 60° C., and 14.42 g of allyl bromide was slowly added dropwise with stirring. After the dropwise addition, the reaction was continued for 6 hours, and the reaction was stopped. Low boilers and solvents were distilled off under reduced pressure, and the fraction at 148-150°C (1.33Kpa) was collected to obtain 15.22g of colorless transparent liquid 2,2-dimethylol propionate allyl alcohol, with a yield of 87.7%
[0026] Hydroxyl protection reaction (molar ratio 2,2-dimethylol propionate: hexamethyldisilazane=1:1.5)
[0027] Into a 100 mL three-necked flask, 10.03 g of allyl 2,2-dimethylol propionate was added. At room temperature, 14.25 g of hexamethyldisilaz...
Embodiment 2
[0035] In the esterification reaction of Example 1, the ratio of the amount of 2,2-dimethylolpropionic acid and allyl bromide is changed to 1:1.5, potassium carbonate is changed to sodium carbonate, the reaction temperature is changed to 80°C, and the reaction The time was changed to 10 h, and other reaction conditions were as described in Example 1 to obtain allyl 2,2-dimethylol propionate with a yield of 84%.
Embodiment 3
[0037] The ratio of the amount of 2,2-dimethylolpropionic acid and allyl bromide in the esterification reaction of Example 1 is changed to 1:1, and other reaction conditions are as described in Example 1 to obtain 2,2 - allyl dimethylol propionate, the yield is 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com