Method for preparing 8-hydroxyquinoline metal compounds
A hydroxyquinoline and compound technology, which is applied in the field of preparation of high-purity 8-hydroxyquinoline metal compounds, can solve the problems of many side reactions, low yield of 8-hydroxyquinoline, long production cycle and the like, and achieves improved production. efficiency and purity, avoid side reactions, and prevent environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
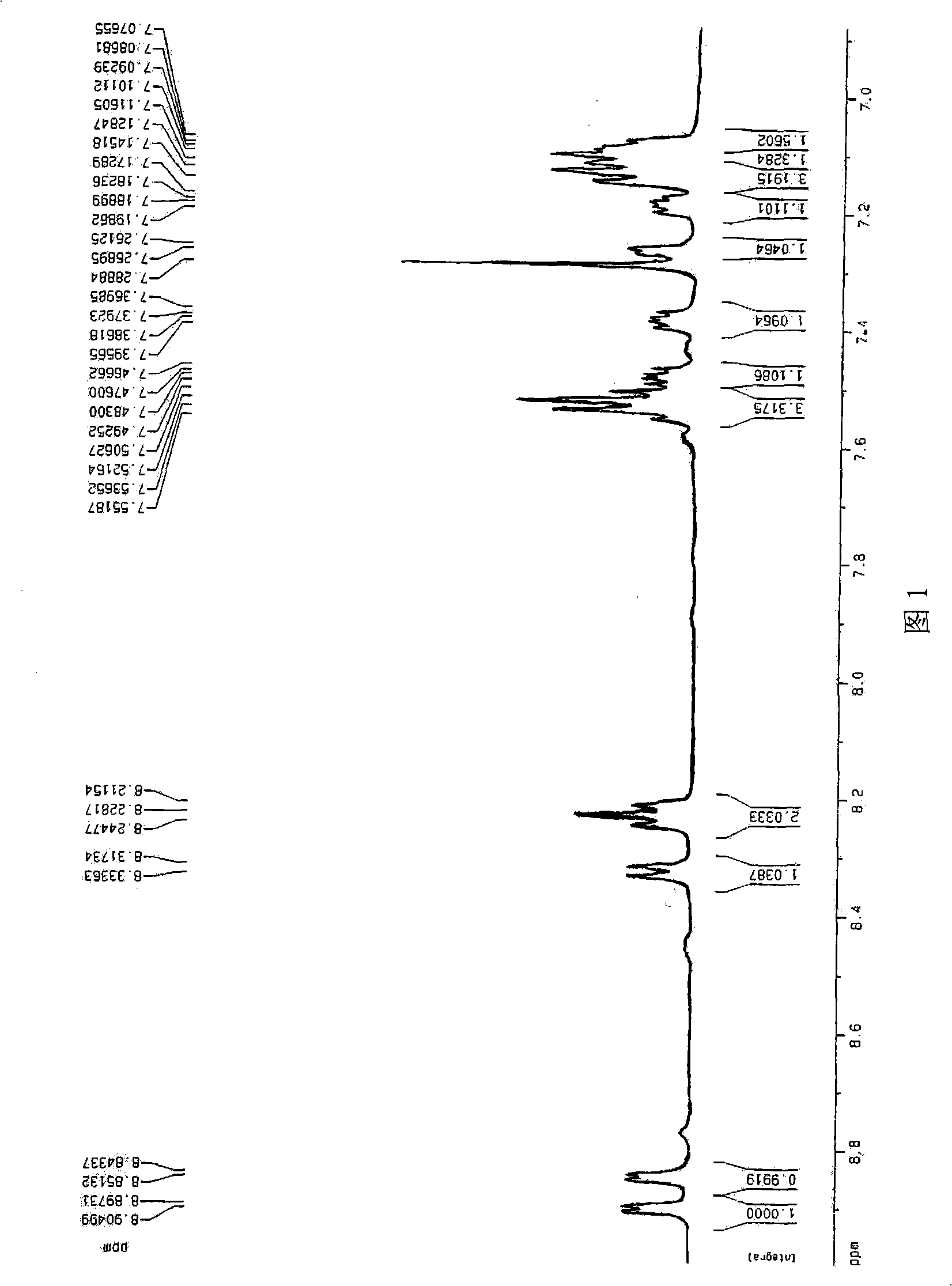
[0031] Embodiment 1: Aluminum isopropoxide is used as raw material to synthesize 8-hydroxyquinoline aluminum
[0032] Add 120mL toluene to a 500mL four-necked bottle, add aluminum isopropoxide to a four-necked bottle, fill with nitrogen and stir, completely dissolved, colorless and transparent. 8-Hydroxyquinoline was dissolved in 120mL toluene, added to the reaction through a constant pressure dropping funnel, and 8-Hydroxyquinoline solution was added dropwise, yellow-green color was produced immediately, and yellow precipitate was formed soon, and the 8-Hydroxyquinoline solution was completely added A large amount of precipitation was produced. The temperature was maintained at 60°C, and the heating was stopped after 30 minutes of reaction, cooled to room temperature, and filtered to obtain a large amount of fibrous solid. Wash twice with 150x2mL toluene; wash twice with 150x2mL petroleum ether, and dry in vacuum at 70°C for 6 hours to obtain 17.5 g of a bright yellow produc...
Embodiment 2
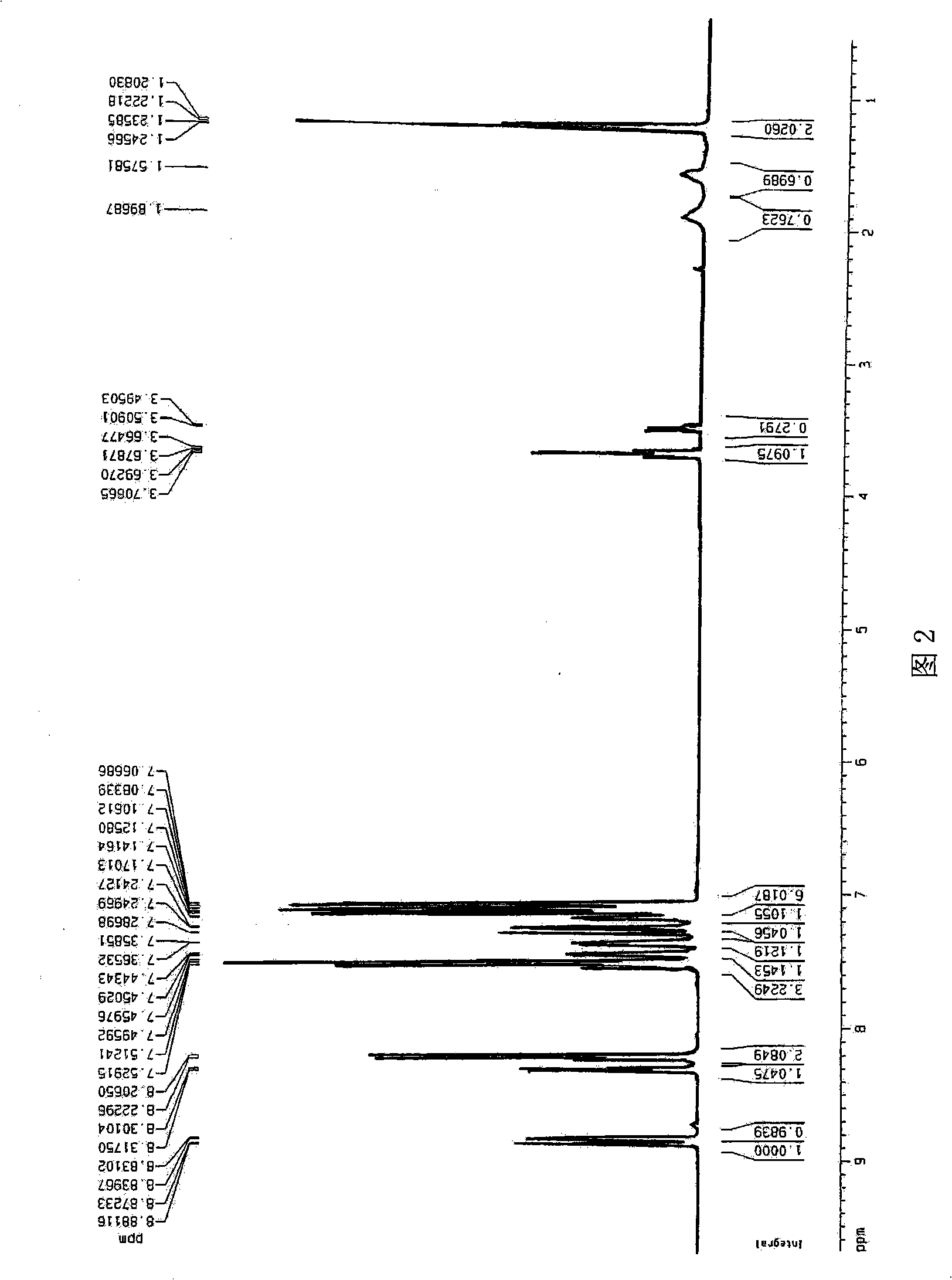
[0037] Embodiment 2: Synthesis of two (2-methyl-8-hydroxyquinoline)-4-(phenylphenoxy)aluminum
[0038] Add 4-phenylphenol and 200ml of toluene into a 1L four-neck flask, fill with nitrogen and stir to completely dissolve. Dissolve aluminum isopropoxide in 120ml of toluene, add dropwise, white turbidity will appear immediately, complete addition in 1.5 hours, and stir for 1 hour. When 2-methyl-8-hydroxyquinoline was added dropwise, yellow-green turbidity immediately appeared. It took 1 hour to complete the addition, and refluxed for 16 hours after the addition. A slightly yellow solid precipitated after cooling, and was filtered to obtain a white solid. Purified with toluene and dried, the yield was 80%.
Embodiment 3
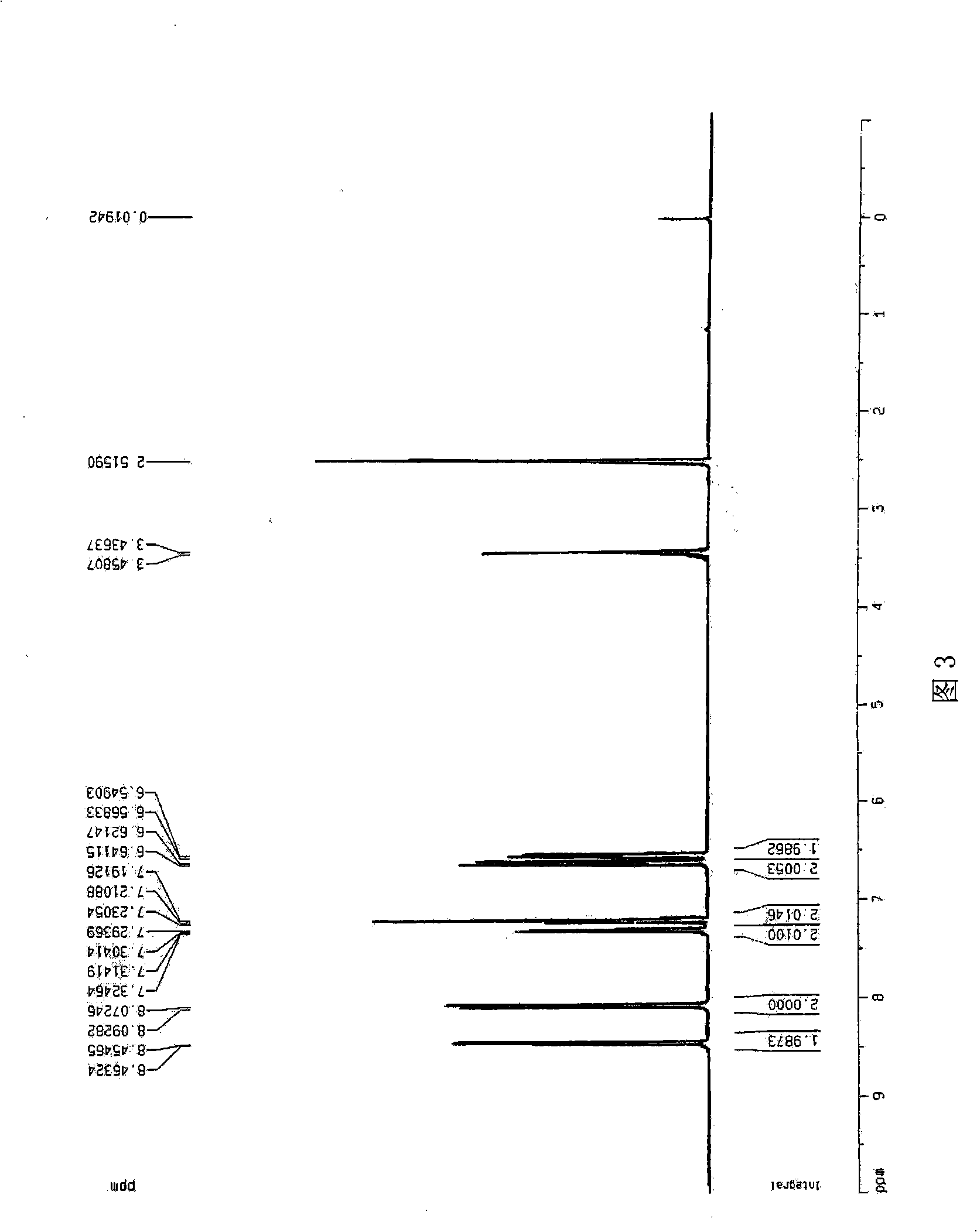
[0039] Embodiment 3: 8-hydroxyquinolate lithium is synthesized
[0040] Add 120ml of dichloromethane into a 250ml four-necked bottle, first add hydrated lithium hydroxide, and stir until it becomes white and turbid. 8-Hydroxyquinoline was added in batches, and milky yellow was produced in less than half a minute; soon the milky yellow increased, and reacted overnight at 25°C to obtain a large amount of light yellow solid. A large amount of milky yellow solid was obtained by filtration, washed twice with 100 ml of dichloromethane at 200°C for 24 hours, and the yield was 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com