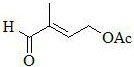
A kind of preparation method of high yield 2-methyl-4-acetoxy-2-butenal
A technology of acetoxy and crotonaldehyde, applied in the field of medicinal chemistry, can solve the problems of unsuitable and simple operation, low substrate activity, high equipment requirements, etc., and achieve the effect of suitable reactivity, easy operation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
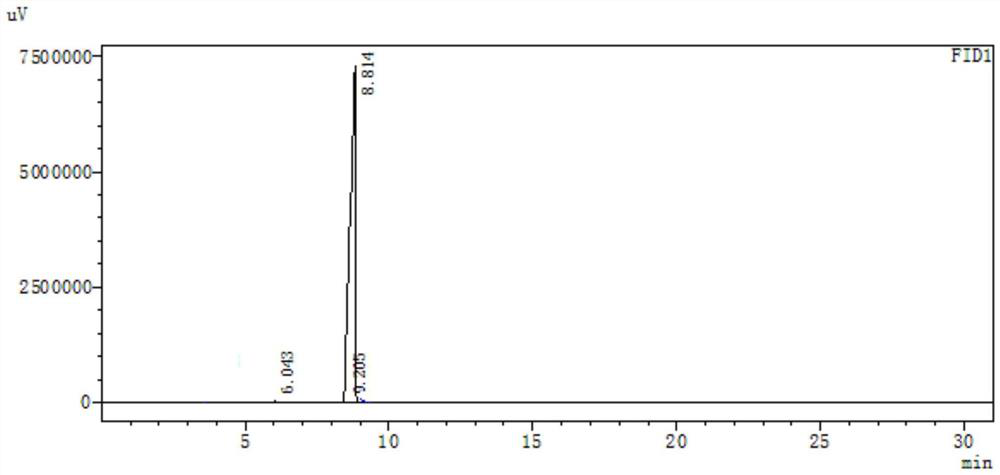
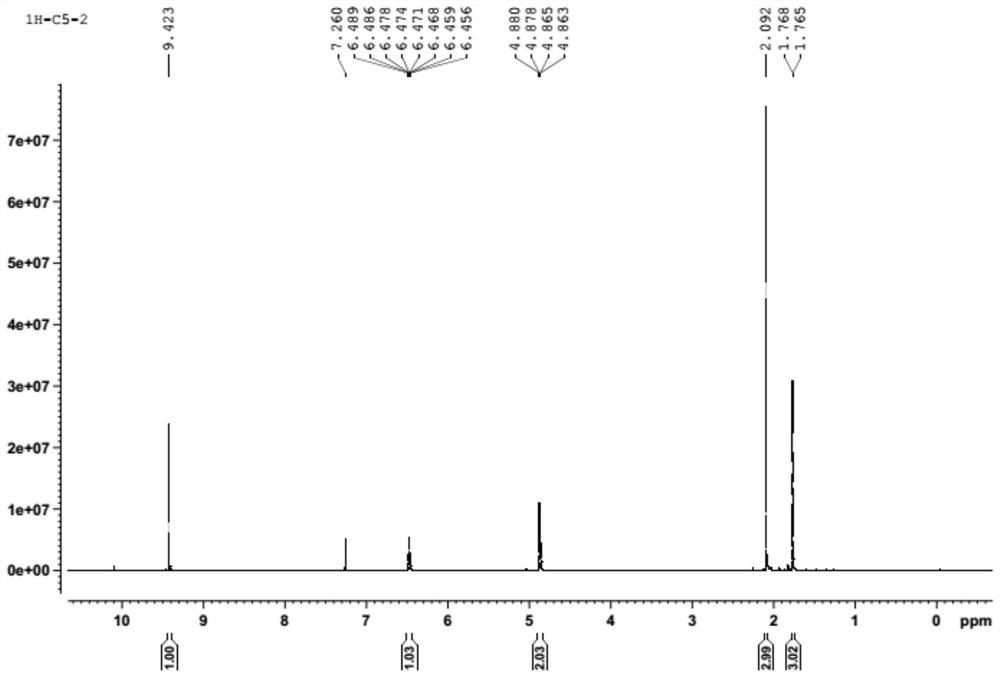
Image
Examples
Embodiment 1
[0076] Example 1: Preparation of 3-formyltetrahydrofuran (III)
[0077] To a 500 ml stainless steel autoclave with stirring and a thermometer, add 70.0 g (1.0 mol) 2,5-dihydrofuran (II), 140 g toluene, 0.5 g tris(triphenylphosphine) rhodium carbonyl hydride, 0.5 g g of triphenylphosphine, closed the autoclave, replaced the gas in the autoclave 3 times with nitrogen, and then introduced the synthesis gas CO / H 2 (volume ratio 1:1), keep the synthesis gas pressure at 4.0-5.0MPa, turn on stirring, be warming up to 90-95°C, react for 4 hours, cool down, replace with nitrogen 3 times, remove the reaction liquid, wash the reaction with 30 grams of toluene The catalyst was removed by filtration, the filtrate was distilled to recover toluene, and then distilled under reduced pressure (80-90°C / 1-2mmHg) to obtain 94.6 g of 3-formyltetrahydrofuran (III) with a yield of 94.6% and a gas-phase purity of 99.7%.
Embodiment 2
[0078] Example 2: Preparation of 3-formyltetrahydrofuran (III)
[0079] Into a 500 ml stainless steel autoclave with stirring and a thermometer, add 70.0 g (1.0 mol) 2,5-dihydrofuran (II), 180 g toluene, 0.5 g tris(triphenylphosphine) rhodium chloride, 0.6 g g of triphenylphosphine, closed the autoclave, replaced the gas in the autoclave 3 times with nitrogen, and then introduced the synthesis gas CO / H 2 (volume ratio 1:1), keep the synthesis gas pressure at 5.0-6.0MPa, turn on stirring, be warming up to 100-105°C, react for 4 hours, cool down, replace with nitrogen 3 times, remove the reaction liquid, wash the reaction with 30 grams of toluene The catalyst was removed by filtration, the filtrate was distilled to recover toluene, and then distilled under reduced pressure (80-90°C / 1-2mmHg) to obtain 93.8 g of 3-formyltetrahydrofuran (III) with a yield of 93.8% and a gas-phase purity of 99.6%.
Embodiment 3
[0080] Example 3: Preparation of 2-formyl-4-acetoxy-1-butene (IV)
[0081] Into a 500-milliliter four-necked flask connected with a stirring, thermometer and reflux condenser, add 200 g of toluene, 50.0 g (0.5 mol) of 3-formyltetrahydrofuran (III) prepared in Example 1, 48.0 g (0.8 mol) of acetic acid , 0.20 g of p-toluenesulfonic acid, and the reaction was stirred at 110-112 ° C for 5 hours. Cooled to 20 to 25°C, changed to a distillation system, first distilled to recover toluene and excess acetic acid, and then changed to high vacuum vacuum distillation (85-100°C / 1-2mmHg) to obtain 68.5 g of 2-formyl-4-acetoxy Base-1-butene (IV), the yield was 96.5%, and the gas-phase purity was 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com