Platinum-cobalt dual-metal catalyst with room-temperature benzene hydrogenation activity and preparation method thereof
A bimetallic catalyst and catalyst technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, hydrogenation hydrocarbon production, chemical instruments and methods, etc., can solve the problems of no patents and literature reports, etc., and achieve high room temperature heating Effects of hydrogen efficiency, energy saving, and process simplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, preparation and activity evaluation of catalyst
[0029] 1. Preparation of catalyst
[0030] 1. Preparation of catalyst precursor
[0031] 0.07g Pt(NH 3 ) 4 (NO 3 ) 2 and 1.5g Co(NO 3 ) 2 ·6H 2 O was dissolved in 100 mL of deionized water, 2.9 g of activated carbon was added, stirred magnetically for 2 h, evaporated to dryness in a water bath at 95 °C, and then dried in an oven at 110 °C for 12 h.
[0032] 2. Preparation of catalyst
[0033] Put 120 mg of the catalyst precursor prepared in step 1 into a U-shaped quartz tube, activate in hydrogen-nitrogen mixed gas at 350° C. for 1 h, and flow both hydrogen and nitrogen at 20 mL / min.
[0034] 2. Catalyst performance evaluation
[0035] 1. Determination of metal specific surface area and CO chemical adsorption capacity of catalyst
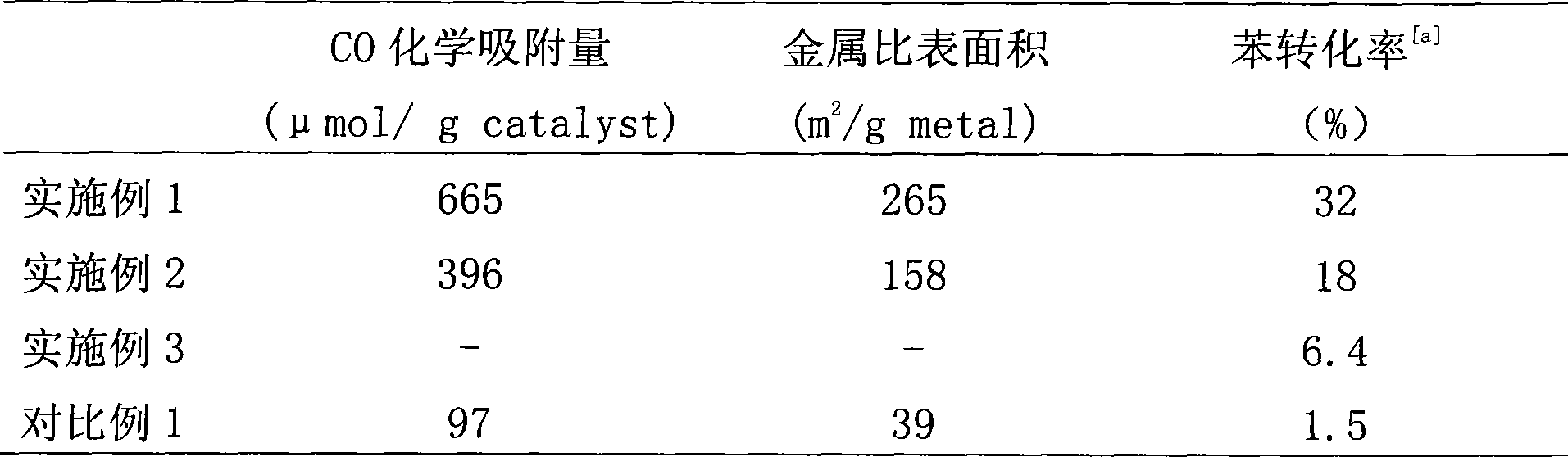
[0036] The metal specific surface area and CO chemisorption capacity of the catalyst prepared in step 1 were measured, and the results are shown in Table 1.
[0037] ...
Embodiment 2
[0040] Embodiment 2, catalyst preparation and activity evaluation
[0041] 1. Preparation of catalyst
[0042] 1. Catalyst precursor preparation
[0043]0.07g Pt(NH 3 ) 4 (NO 3 ) 2 and 1.5g Co(NO 3 ) 2 ·6H 2 O was dissolved in 100 mL of deionized water, 2.9 g of activated carbon was added, stirred magnetically for 2 h, evaporated to dryness in a water bath at 95 °C, and then dried in an oven at 110 °C for 12 h.
[0044] 2. Catalyst activation
[0045] Put 100 mg of the catalyst precursor prepared in step 1 into a U-shaped quartz tube, activate in a hydrogen-nitrogen mixed gas at 500°C for 1 h, and flow both hydrogen and nitrogen at 20 mL / min.
[0046] 2. Catalyst performance evaluation
[0047] 1. Determination of metal specific surface area and CO chemical adsorption capacity of catalyst
[0048] The metal specific surface area and CO chemisorption capacity of the catalyst prepared in step 1 were measured, and the results are shown in Table 1.
[0049] 2. Benzene ...
Embodiment 3
[0052] Embodiment 3, catalyst preparation and activity evaluation
[0053] 1. Preparation of catalyst
[0054] 1. Preparation of catalyst precursor
[0055] 0.07g Pt(NH 3 ) 4 (NO 3 ) 2 and 1.5g Co(NO 3 ) 2 ·6H 2 O was dissolved in 100 mL of deionized water, 2.9 g of activated carbon was added, stirred magnetically for 2 h, evaporated to dryness in a water bath at 95 °C, and then dried in an oven at 110 °C for 12 h.
[0056] 2. Catalyst activation
[0057] 120 mg of the catalyst precursor prepared in Step 1 was put into a U-shaped quartz tube, activated in nitrogen at 500°C for 1 h, and the nitrogen flow rate was 50 mL / min.
[0058] 2. Catalyst performance evaluation
[0059] After step 1 is completed, a reaction gas is introduced into the U-shaped quartz tube, the reaction gas is a hydrogen-nitrogen mixture, and the flow rates are 10 mL / min and 40 mL / min, respectively. Benzene was injected with a micropump at a flow rate of 0.5mL / h, and was brought into the catalyst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com