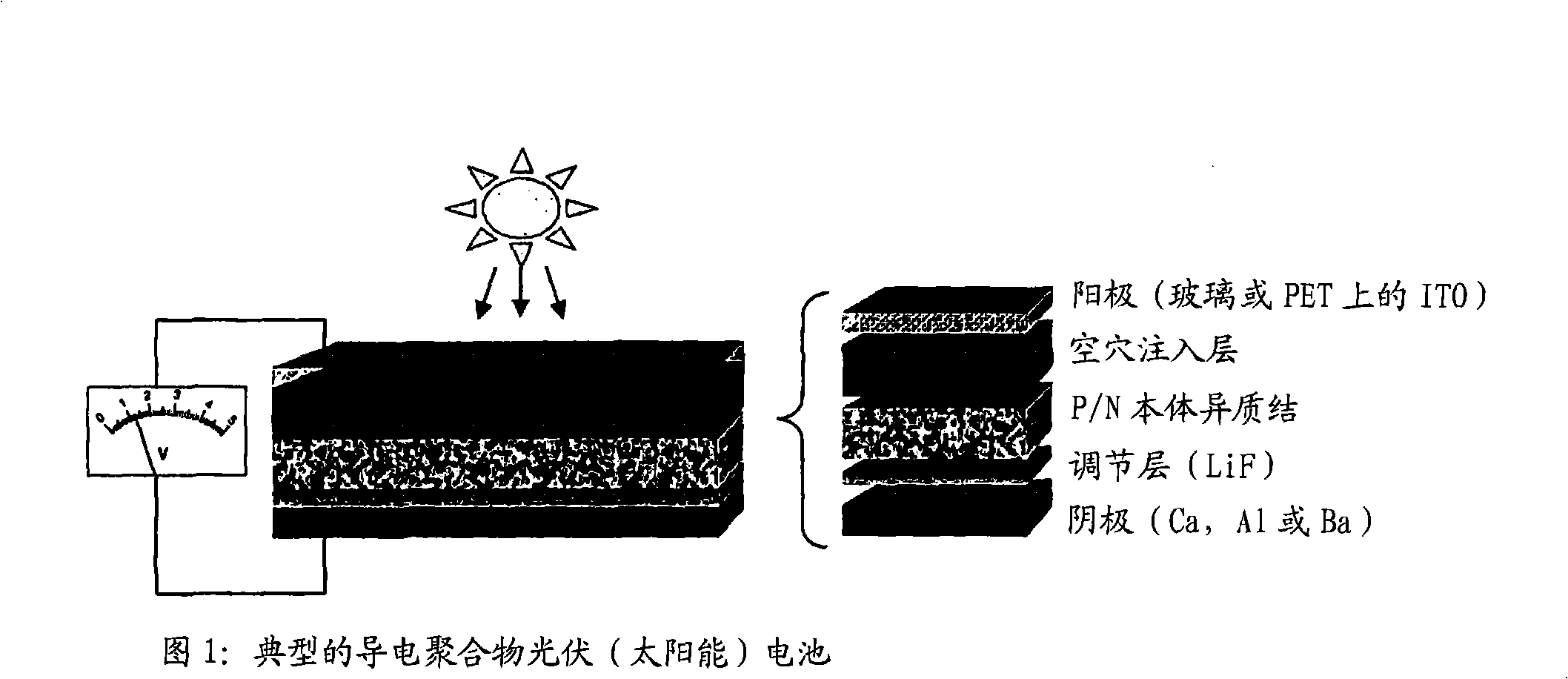
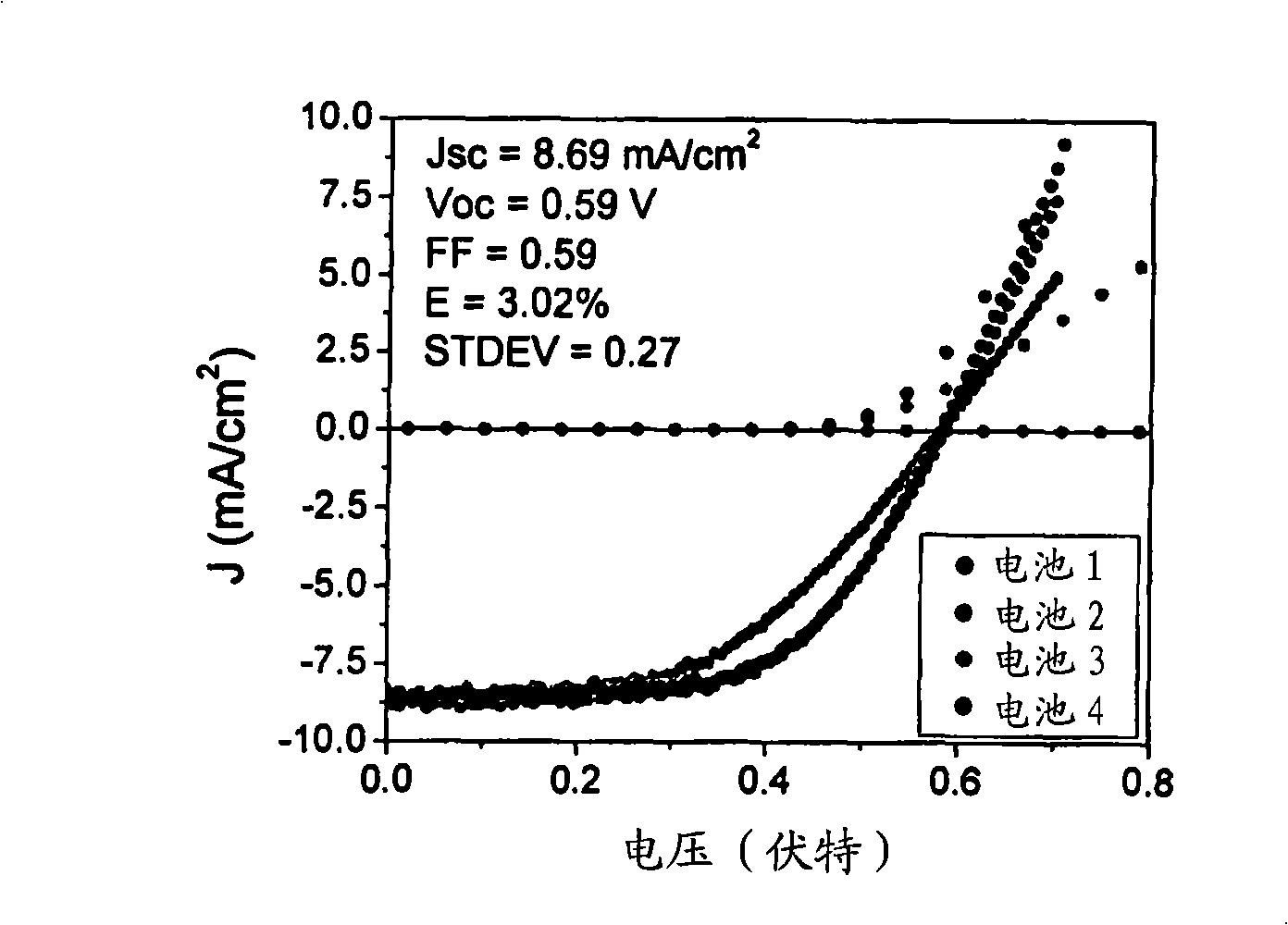
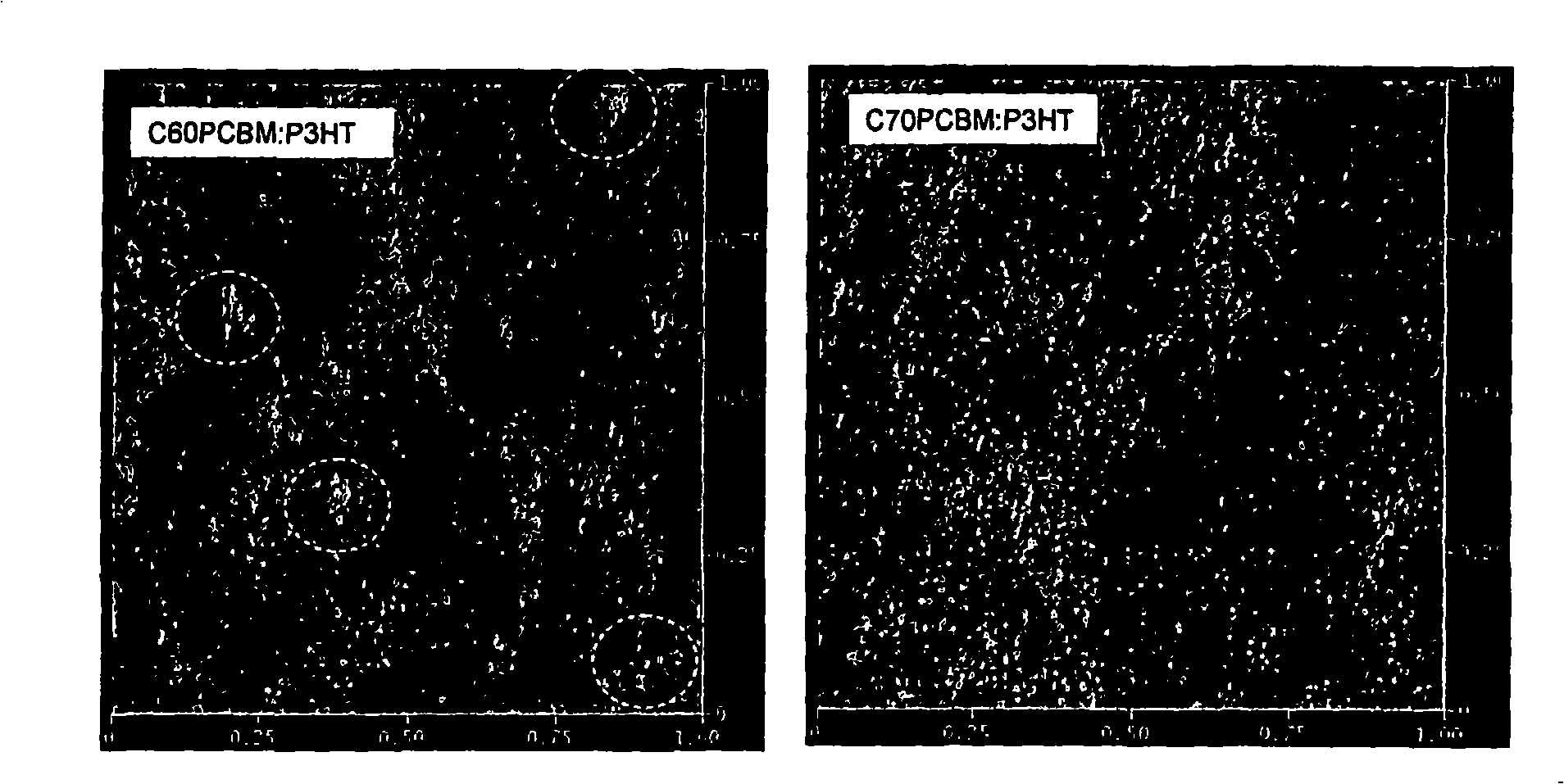
Organic photovoltaic devices comprising fullerenes and derivatives thereof
A fullerene derivative, fullerene technology, applied in photovoltaic power generation, electrical solid-state devices, semiconductor devices, etc., to achieve good photovoltaic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0195] Embodiment 1. A photovoltaic device, which includes:
[0196] The first electrode,
[0197] Second electrode,
[0198] At least one active layer deposited between the first and second electrodes, wherein the active layer includes at least one polythiophene and at least one fullerene derivative containing an electron withdrawing group.
[0199] 2. The device of embodiment 1, wherein the fullerene derivative is a C60 fullerene.
[0200] 3. The device according to embodiment 1, wherein the fullerene derivative is C70 fullerene.
[0201] 4. The device of embodiment 1, wherein the fullerene derivative is C 84 fullerene.
[0202] 5. The device of embodiment 1, wherein the fullerene derivative comprises C60Cl6, C60 (C9H8), C60Br24, C60Cl (CH2ClChC 12).
[0203] 6. The device of embodiment 1, wherein the polythiophene is a stereoregular polythiophene.
[0204] 7. The device of embodiment 1, wherein the polythiophene is a copolymer.
[0205] 8. The device of embodiment 1, further co...
Embodiment 1
[0234] Example 1: C 60 -Synthesis of indene adducts
[0235] As a starting point, use C 60 The indene adduct was synthesized by using the description in the reference (Puplovskis et al., "New Route for [60] Fullerene Functionalization in [4+2] Cycloaddition Reaction Using Idene." Tetrahedron Lett. 1997, 38, 285-288) . Will C 60 To about 6mg mL -1 The concentration is dissolved in o-dichlorobenzene. Indene relative to C 60 It was added in a 12-fold molar excess, and the resulting mixture was refluxed overnight. Most of the solvent evaporates under reduced pressure, and precipitation occurs after the addition of ethanol. The obtained solid was dried, dissolved in toluene, and then used with a Cosmosil Buckyprep analytical column (250 × 4.6 mm, Nacalai Tesque, Inc.) installed on an Agilent 1100 series instrument equipped with a variable wavelength detector operating at 330 nm. ) High pressure liquid chromatography analysis. 1ml min -1 The flow rate of toluene is used for elution....
Embodiment 2
[0236] Example 2: C 70 -Synthesis of Indene Monoadducts
[0237] The C70-indene monoadduct was synthesized according to the method developed for the C60-indene adduct. Will C 70 Dissolved in o-dichlorobenzene. After adding indene in a 12-fold molar excess, reflux was maintained for 8 hours. After reducing the volume under reduced pressure and adding ethanol, the solid was recovered, dried and redissolved in toluene. HPLC analysis using the same steps as above showed that there are mainly monoadducts, which may be due to the difference with C 60 -Compared with the synthesis of adducts, the reaction time is reduced. Purification using flash chromatography makes C 70 -The monoadducts are separated with a purity of 98.6%. The corresponding HPLC chromatogram is given below. Identified means C 70 Two main isomers with different addition sites on the cage.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com