Dimethyldiguanide of the thiazolidinedione pharmaceutical, preparation method and use thereof
A technology of thiazolidinedione and metformin, applied in the field of salt or adduct
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Synthesis of metformin free base
[0026] In the round bottom flask, add metformin hydrochloride 4.99g (0.03mol), add dehydrated alcohol 200ml, add the sodium ethylate dehydrated alcohol solution of equimolar under the situation of stirring again, stir overnight at room temperature, filter off the precipitate that produces, Ethanol was distilled off from the filtrate under reduced pressure to obtain a solid, which was dried under reduced pressure at room temperature to obtain 3.92 g of metformin free base.
Embodiment 2
[0028] Synthesis of Rosiglitazone Metformin Salt (Adduct)
[0029] Take 2.27g (0.006mol) of rosiglitazone sodium and 1.00g (0.006mol) of metformin hydrochloride, put them in a round bottom flask; add 120ml of absolute ethanol, heat to reflux while stirring, after reflux for about 10 minutes, filter while hot , the filtrate was distilled off part of the ethanol under reduced pressure and then cooled to room temperature, a large amount of off-white solid precipitated, recrystallized with absolute ethanol to obtain 2.26 g of white solid, yield 77%, mp; 182-184 ° C.
[0030] Take 2.14g (0.006mol) of rosiglitazone free base and 0.78g (0.006mol) of metformin free base and put them in a round-bottomed flask, add 70ml of ethanol and heat to reflux for about 5 minutes, cool, and recrystallize the precipitated solid with ethanol to obtain a white Crystalline solid 2.16g, yield 74%, melting point 182-184°C
Embodiment 3
[0032] Synthesis of Pioglitazone Metformin Salt (Adduct)
[0033] Take 1.00g of metformin hydrochloride, add 50ml of ethanol, heat and reflux to dissolve, then add 2.28g of pioglitazone sodium dissolved and clarified with 50ml of absolute ethanol, and sodium chloride precipitates immediately. After evaporation under reduced pressure, a white solid was obtained, which was recrystallized from a mixed solvent of ethanol / ethyl acetate to obtain 2.28 g of a white solid, with a yield of 78% and a melting point of 172-174°C.
[0034] Get 2.14g (0.006mol) of pioglitazone free base and 0.78g (0.006mol) of metformin free base in a round-bottomed flask, add 50ml of ethanol and heat to reflux. After the solution becomes clear, ethanol is distilled off under reduced pressure to obtain a white solid. Recrystallized with ethanol / ethyl acetate to obtain 2.37g of white crystalline solid, yield 81%, melting point 172-174°C
[0035] Example 3
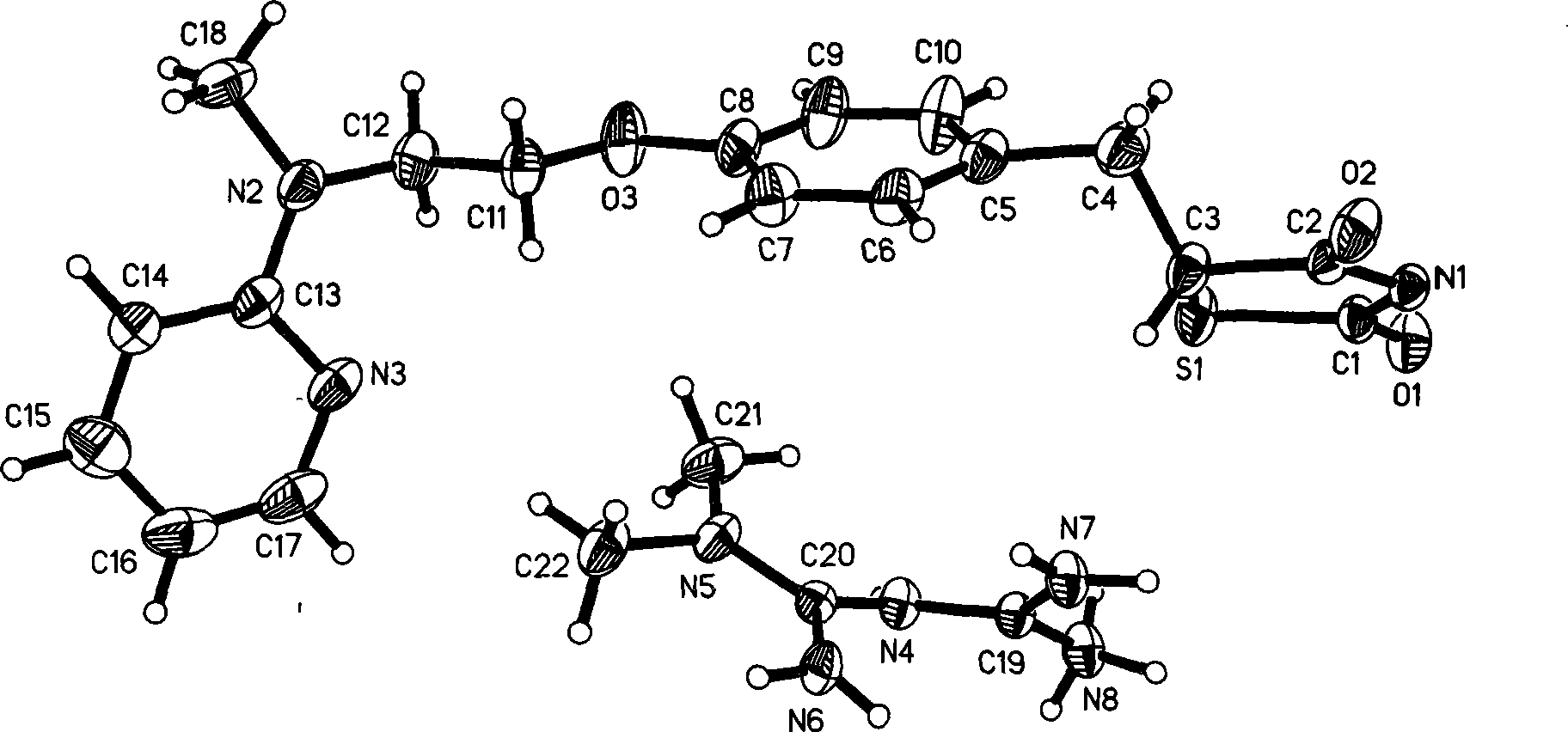
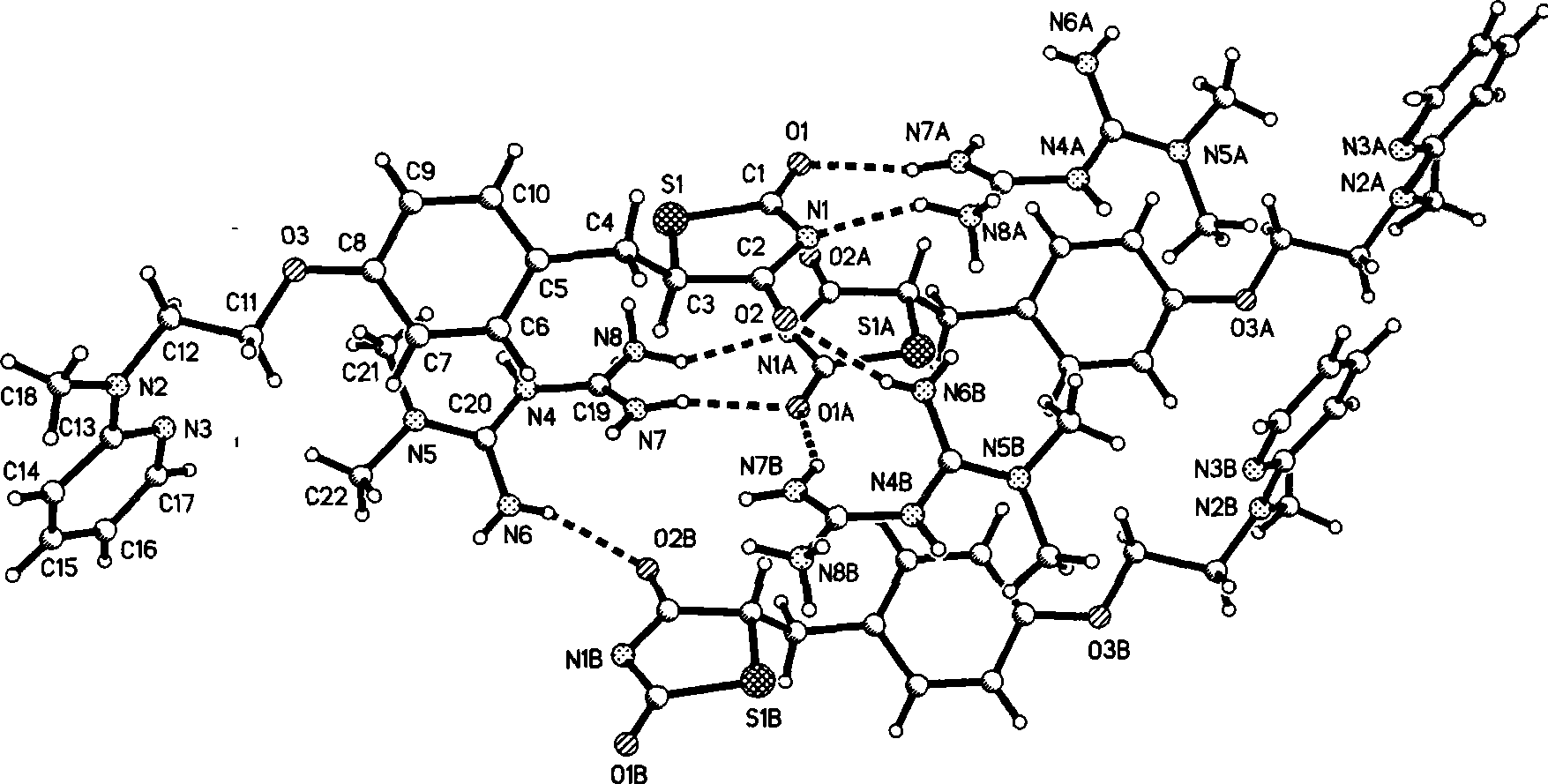
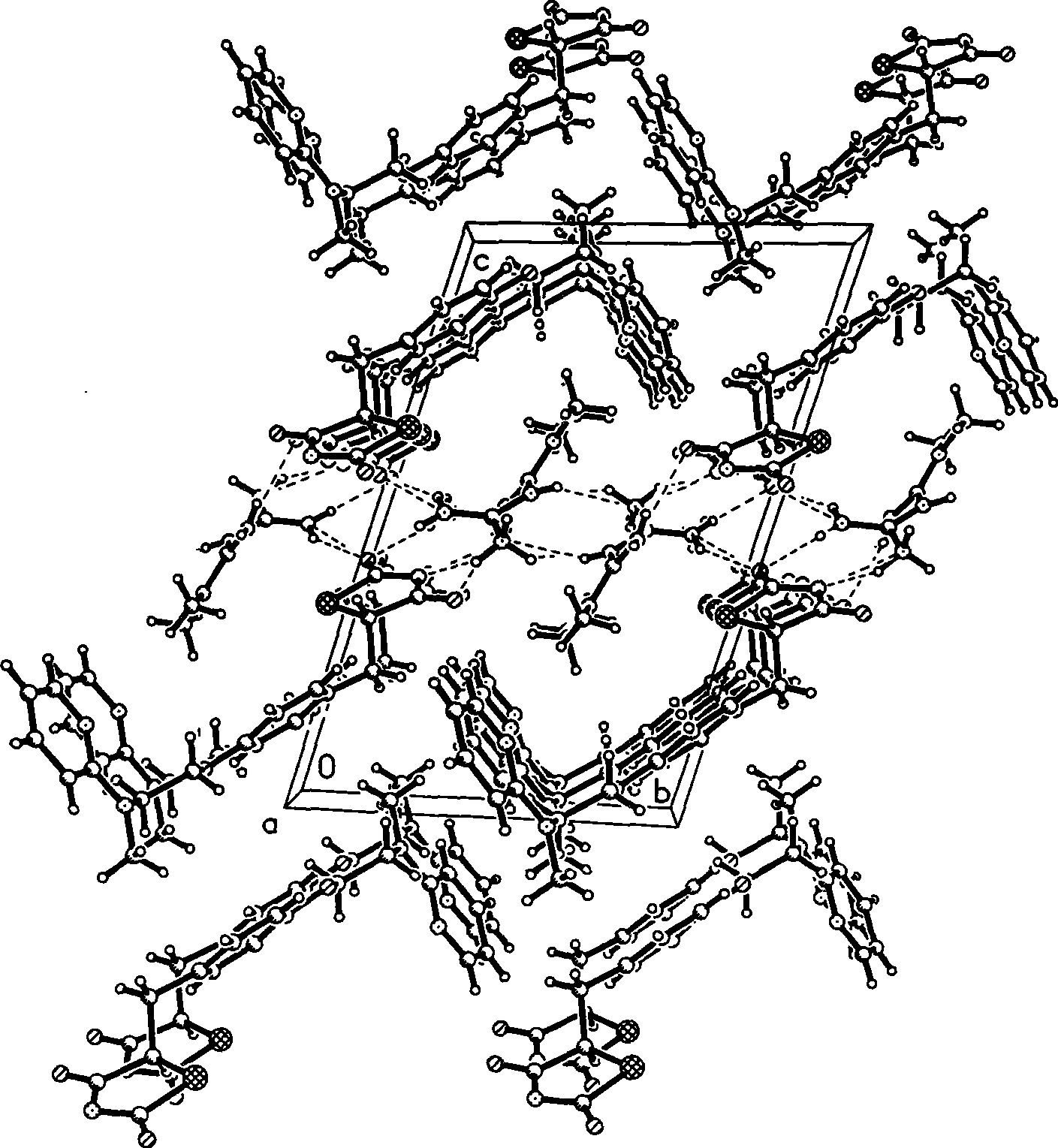
[0036] Growth and Single Crystal Structure of Ros...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com