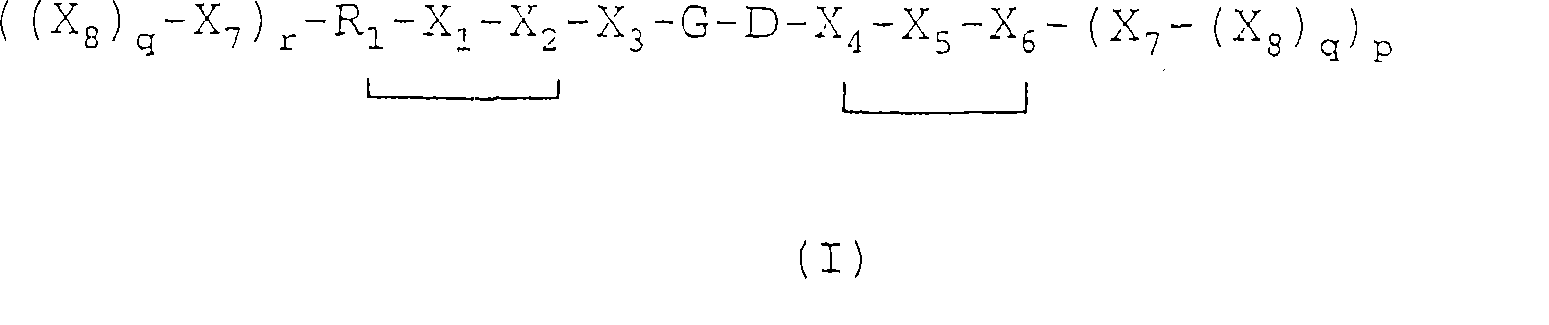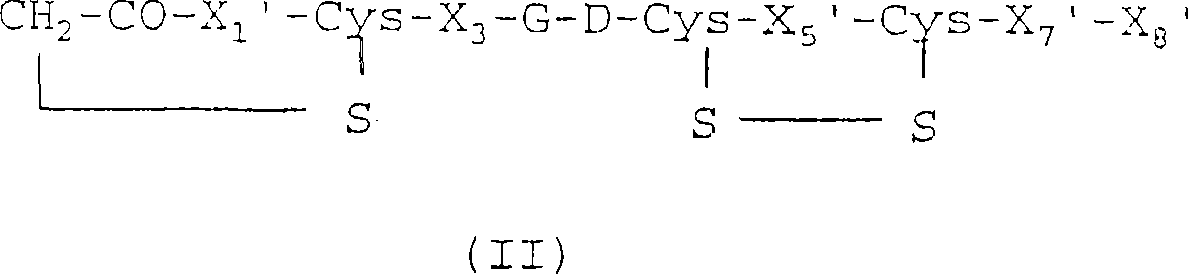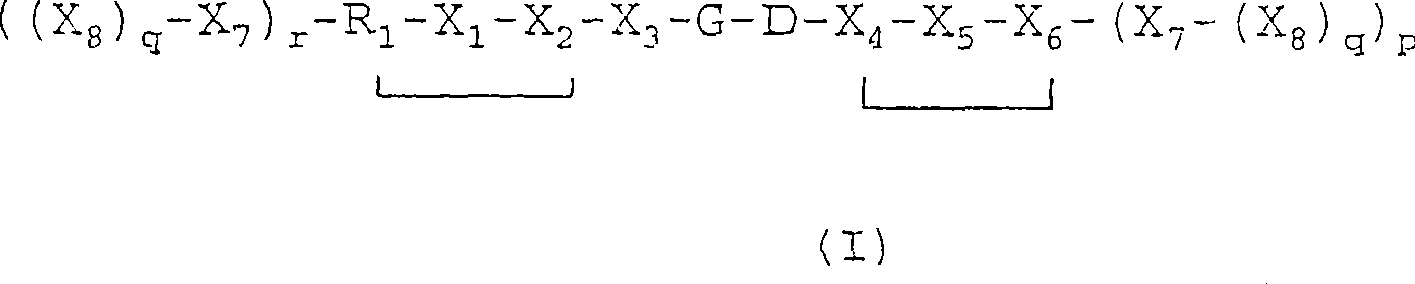Peptide-based compounds
A compound and chelate technology, applied in the field of new peptide-based compounds, can solve the problem of high background noise
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0054] Compound 1:
[0055]
[0056] Compound 2: Vehicle
[0057]
[0058] Compound 3: Examples of V-L-R Compounds of Formula I
[0059]
[0060] Compound 4: Example of a compound of formula II
[0061]
[0062] Compound 5: able to connect, for example 99m The formula of Tc? compound example
[0063]
[0064] In most cases, it is preferred that the amino acid in Vehicle V is in the L-form. However, in some embodiments of the invention, one, two, three or more amino acids in vehicle V are preferably in the D-form. The presence of this D-form amino acid has an important effect on the serum stability of the vehicle. In this regard, particular attention is paid to the X 1 A vehicle with D-tyrosine at the site.
[0065] The present invention also provides a pharmaceutical composition comprising an effective amount (for example, an amount effective for enhancing image contrast and / or treatment in vivo imaging) of a compound of general formula (I) or an acid ad...
Embodiment 1
[0144] Embodiment 1: the synthesis of compound 1
[0145]
[0146] Synthesis of Technetium Chelate-Pn216
[0147] a) Chloro-nitroso intermediate (3-chloro-3-methyl-2-nitrosobutane)
[0148] A mixture of 2-methylbut-2-ene (18.5ml) and isoamyl nitrate (19.5ml) was stirred, cooled to -10°C and concentrated hydrochloric acid (17.5ml) was added carefully keeping the temperature below 0°C. The reaction mixture was stirred at this temperature for 30 minutes. The formed precipitate was collected by filtration, washed with 4 x 5 ml ethanol (-20°C) and dried in vacuo to give 3-chloro-3-methyl-2-nitrosobutane as a white solid.
[0149] b) Pn216-(3,3,11,11-tetramethyl-7-aminoethyl-4.7,10-triazatridecane -2,12-diketodioxime)
[0150]To a solution of tris-(2-aminoethyl)amine in acetonitrile (20ml) was added sodium bicarbonate (2.2g, 26mmol). A solution of 3-chloro-3-methyl-2-nitrosobutane (1.8 g, 13 mmol) in dry acetonitrile was added slowly at 0°C. The reaction mixture was ...
Embodiment 2
[0172] Example 2: [Cys 2-8,8-10 ]analog
[0173] a) Synthesis of ClCH 2 CO-Lys-Asp-Cys-Arg-Gly-Asp-Cys(tBu)-Phe- Cys(tBu)-Gly-Gly-OH
[0174]
[0175] Peptides were synthesized on an ABI 433A automated peptide synthesizer using a 1 mmol amino acid cartridge in the order of 0.25 mmol using Fmoc-Gly wang resin (NoVabiochem) as starting material. Amino acids were preactivated with HBTU prior to coupling. Final N-terminal chloroacetylation was performed using chloroacetic anhydride in DMF for 30 minutes.
[0176] Containing TIS (5%), H 2 Both peptide and side chain protecting groups (except tBu) were removed from the resin in TFA of O (5%) and phenol (2.5%) for 2 hours.
[0177] After the above treatment, 260 mg of crude peptide was obtained (analytical HPLC: gradient, 5-50% B over 10 minutes, where A=H 2 O / 0.1%TFA and B=CH 3 CN / 0.1% TFA; column, Phenomenex Luna 3μC18 (2) 50 x 4.6mm; flow rate, 2ml / min, detection, UV214nm; product retention time, 6.5 minute...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com