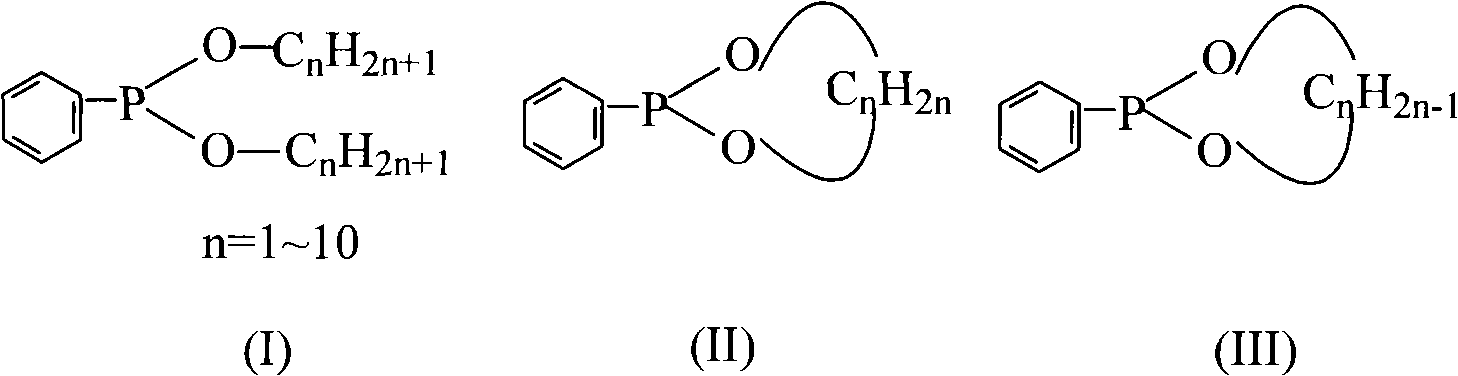
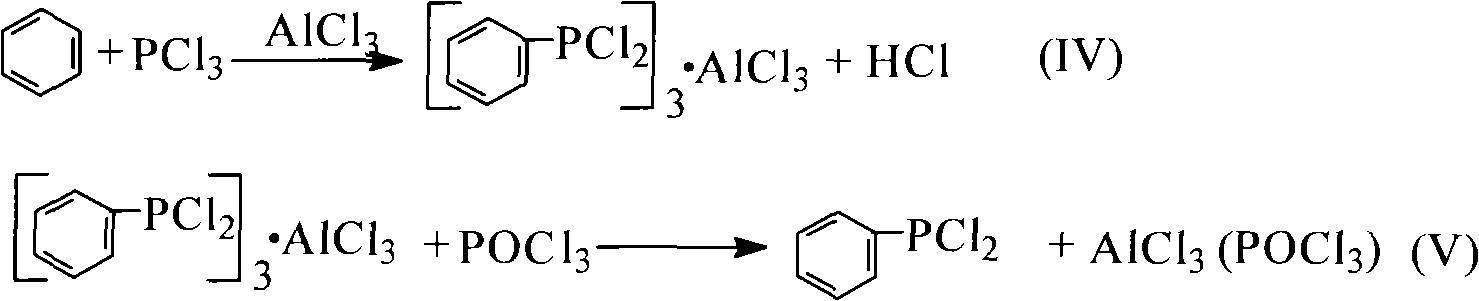Method for preparing dialkyl phenyl-phosphonite
A technology of dialkyl phenyl phosphinate and dialkyl phenyl phosphinate, which is applied in the field of intermediate preparation, can solve the problems of difficult separation, high cost, and easy formation of yellow by-products, etc., so as to improve the conversion The effect of increasing the rate and yield and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of embodiment 1 dimethyl phenylphosphonite
[0040] (1) Synthesis of phenylphosphine dichloride
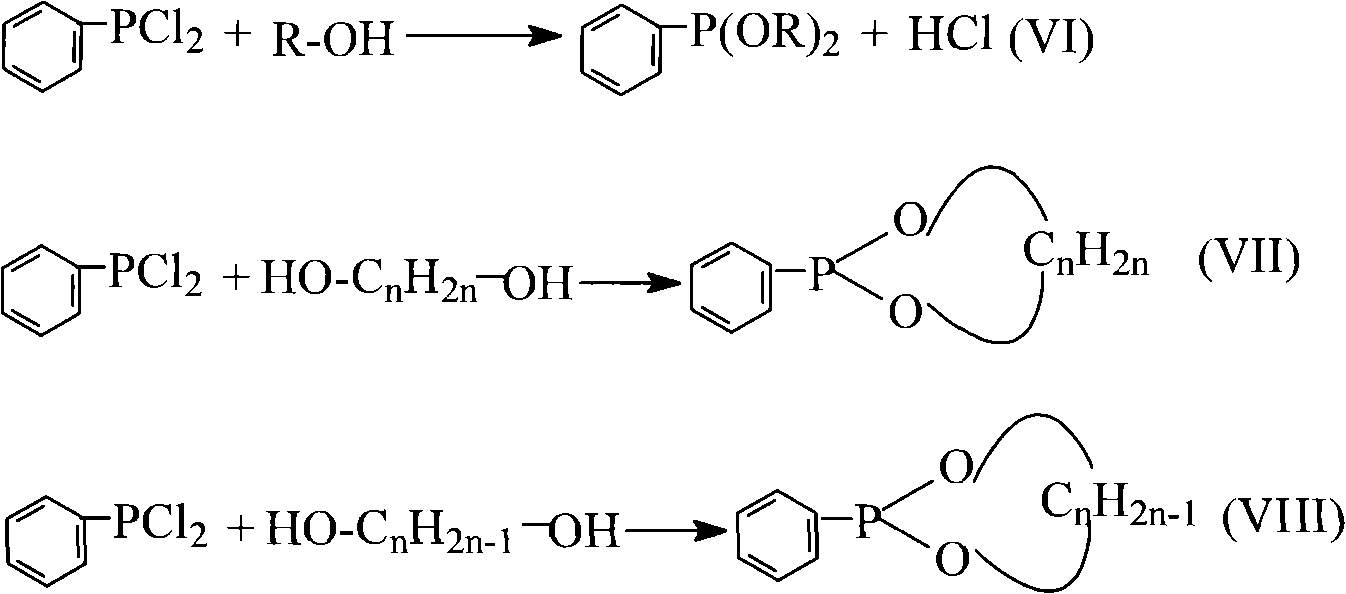
[0041] Add 0.1mol of benzene and 0.3mol of phosphorus trichloride into a 250mL four-necked flask equipped with an electric liquid-sealed stirring device, a constant pressure dropping funnel, a thermometer, and a spherical condenser (the upper part is equipped with a drying tube and a gas absorption tube). , Aluminum trichloride 0.14mol, reflux reaction under stirring until no hydrogen chloride is released (about 5h of reaction). After the reaction was stopped, 0.13 mol of phosphorus oxychloride was evenly added dropwise from the constant pressure funnel to the four-neck flask under stirring, and the dropping time was controlled at 30 min. Then add 45mL petroleum ether, stir and reflux for 30min. After cooling to room temperature, filter under reduced pressure, distill petroleum ether from the filtrate under normal pressure, and distill under reduced pressure to ...
Embodiment 2
[0045] The synthesis of embodiment 2 diethyl phenyl phosphinates
[0046] (1) The synthesis of phenylphosphine dichloride is the same as the first step of Example 1.
[0047] (2) Synthesis of diethyl phenylphosphonite
[0048] Add 0.4mol absolute ethanol to a 250ml four-necked flask equipped with an electric liquid-sealed stirring device, a constant pressure dropping funnel, a thermometer and a spherical condenser (drying tube is installed on the upper part). Add 0.1mol phenylphosphine dichloride dropwise to the constant pressure dropping funnel under stirring, utilize ice-water bath and adjust the rate of addition of phenylphosphine dichloride, control the reaction temperature at about 0°C, after the dropwise addition, within about Stir at 45°C for 2.5h. After the reaction was completed, the reaction solution was poured into a beaker to cool, and filtered to obtain a solid crude product of diethyl phenylphosphinate, which was recrystallized from a mixed solution of petroleum ...
Embodiment 3
[0049] The synthesis of embodiment 3 phenylphosphonite ethylene glycol
[0050] (1) The synthesis of phenylphosphine dichloride is the same as the first step of Example 1.
[0051] (2) Synthesis of ethylene glycol phenylphosphonite
[0052] Add 0.1 mol of anhydrous ethylene glycol to a 250 ml four-necked flask equipped with an electric liquid-sealed stirring device, a constant pressure dropping funnel, a thermometer and a spherical condenser (with a drying tube on the upper part). Add 0.1 mol of phenylphosphine dichloride dropwise to the constant pressure dropping funnel under stirring, control the reaction temperature at about 5°C by using an ice-water bath and adjusting the addition rate, and stir at about 45°C for 3 hours after the dropwise addition. After the reaction was completed, the reaction solution was poured into a beaker to cool, and filtered to obtain a solid crude product of ethylene phenylphosphinate, which was recrystallized from a mixed solution of petroleum ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com