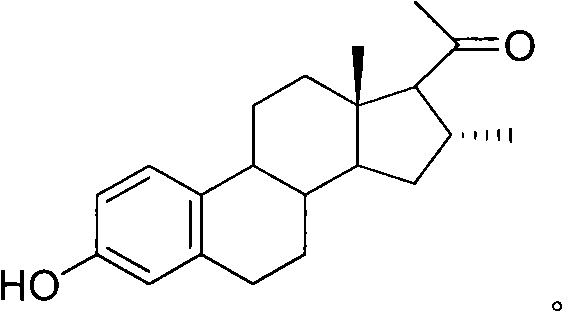
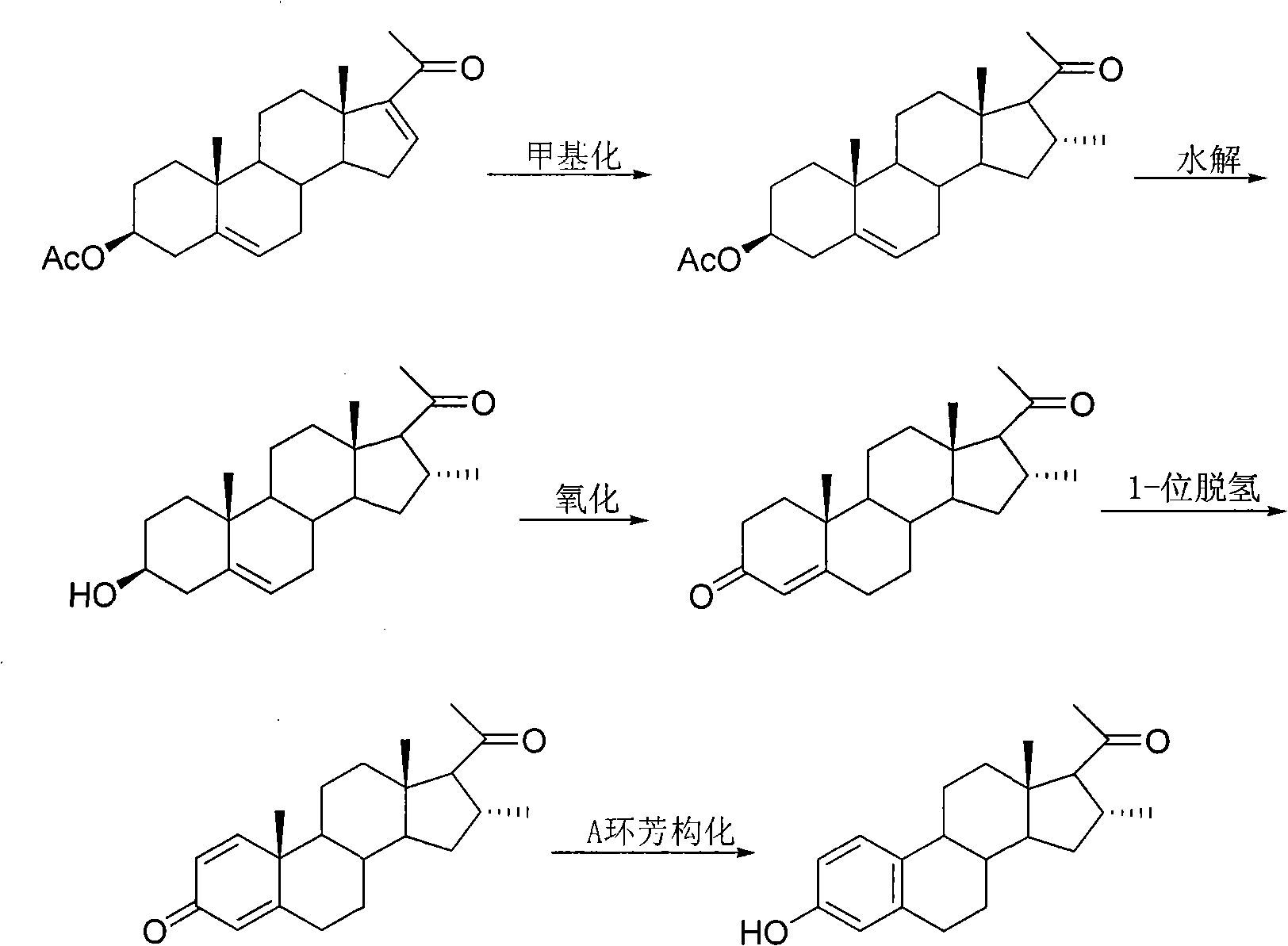
16alpha-methyl-3-hydroxyl-19-norpregnane-1, 3, 5-triene-20-ketone and preparation method thereof
A technology of norpregnantine and methyl group is applied in the field of organic compounds and their preparation, can solve the problems of low reaction yield, high price of estrone, harsh reaction conditions and the like, and achieves good stereoselectivity, high yield, Inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0020] Example: Method for preparing 16α-methyl-3-hydroxy-19-norpregna-1,3,5-trien-20-one
[0021] The first step of methylation
[0022] Under the protection of nitrogen, put 35.6g 3-acetoxy-pregn-5,16-dien-20-one, 1g cuprous bromide, 42.2g trimethylaluminum solution and 13g trimethylchlorosilane into 200g dry in tetrahydrofuran and stirred at room temperature for 2 hours. Add 50g of water and stir until no bubbles come out. Another 100 g of water was added. Extract twice with 180 g of dichloromethane, combine the organic phases, wash twice with 150 g of saturated brine, dry over anhydrous sodium sulfate, and filter. The filtrate was rotary evaporated to dryness and recrystallized from ethyl acetate to obtain 32.0 g of 16α-methyl-3-acetoxy-pregn-5-en-20-one with a yield of 86%. 1 HNMR (500Hz, CDCl 3 ): δ0.66(s, 3H), 0.95(d, 3H, J=6.9Hz), 1.02(s, 3H), 2.04(s, 3H), 2.12(s, 3H), 4.61(m, 1H) , 5.38(d, 1H, J=2.8Hz); 13 CNMR (100MHz, CDCl 3 ): δ209.3, 170.5, 139.6, 122.3, 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com