Liver, spleen specific positive magnetic nuclear resonance contrast agent and method of preparing the same
A nuclear magnetic resonance and contrast agent technology, applied in the field of medical materials, can solve problems such as affecting the accuracy of MRI diagnosis, unsatisfactory blood compatibility, poor biocompatibility, etc., and achieve good dispersion stability, good biocompatibility, The effect of high relaxation properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
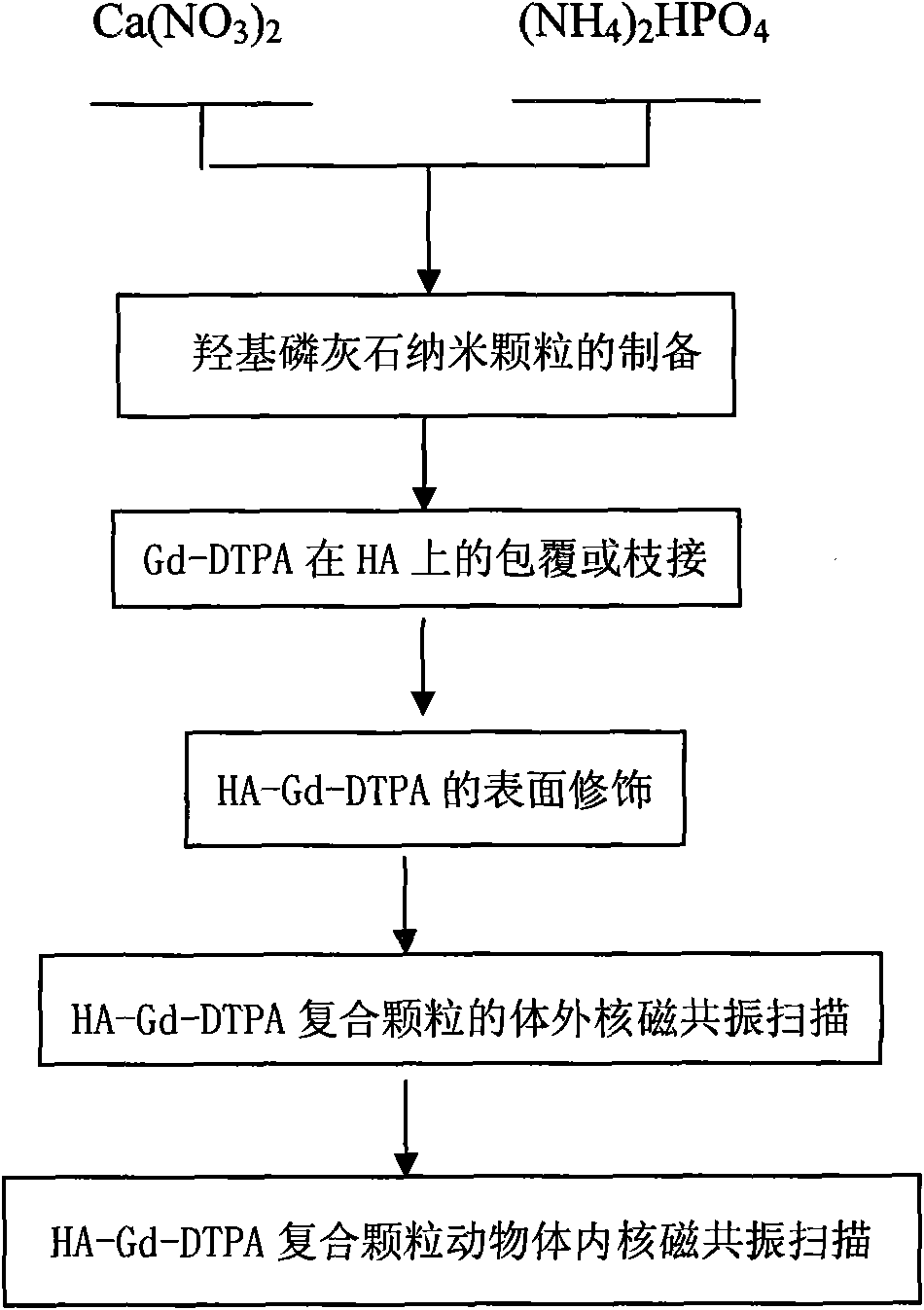
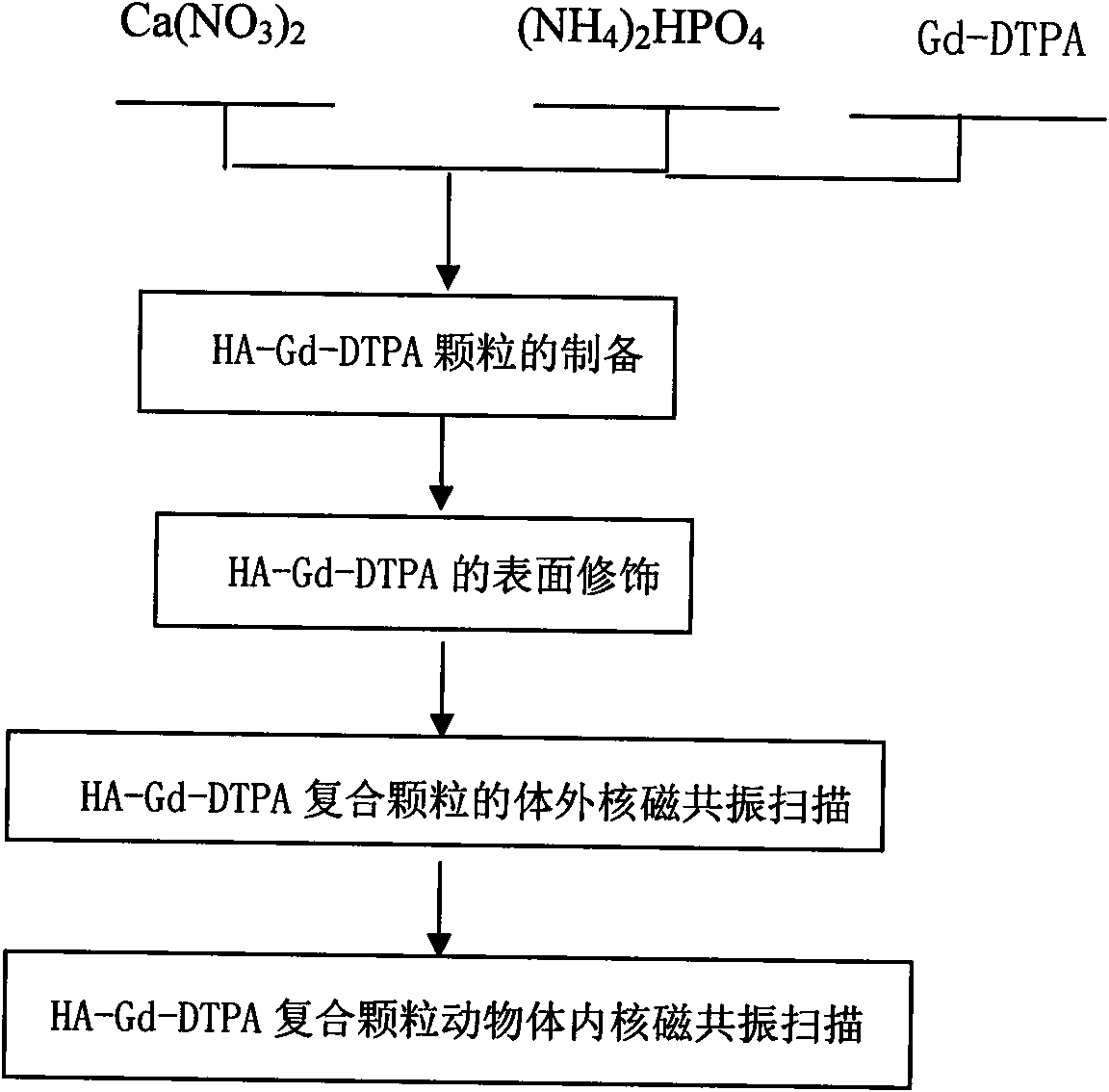
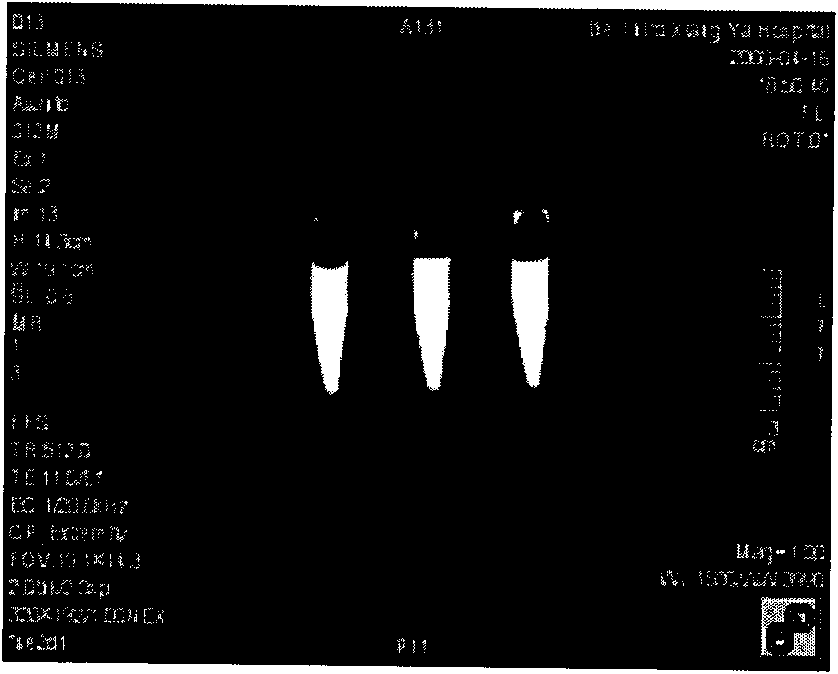
[0032] Weigh 0.1mol Ca(NO 3 ) 2 and 0.06mol(NH 4 ) 2 HPO 4 In a beaker, add 300ml of distilled water to form a solution and stir evenly. Use a dropper to dissolve (NH 4 ) 2 HPO 4 The solution was dropped into Ca(NO 3 ) 2 solution, use NH throughout the process 4 OH adjusts the pH to keep it at 11-12. After the reaction is complete, pour it into an autoclave for hydrothermal synthesis, set the temperature at 160°C, and hold the temperature for 1 hour. After cleaning, filtering, and drying to obtain hydroxyapatite nanoparticles; HA is made into a solution of 0.01mol / L, and gadopentetate meglumine is added with a weight ratio of HA:Gd-DTPA=1:1 under magnetic stirring (according to GD-DTPA injection concentration conversion), continue stirring for 12 hours, then wash, filter, and dry to obtain HA-Gd-DTPA particles, weigh 0.1gHA-Gd-DTPA and put it into 100ml water, add dispersant PEI (per gram Add 0.001-0.05g) to HA, and ultrasonically disperse for 30min. The obtained so...
Embodiment 2
[0034] Weigh 0.1mol Ca(NO 3 ) 2 and 0.06mol(NH 4 ) 2 HPO 4 In a beaker, add 300ml of distilled water to form a solution and stir evenly. Use a dropper to dissolve (NH 4 ) 2 HPO 4 The solution was dropped into Ca(NO 3 ) 2 solution, use NH throughout the process 4OH adjusts the pH to keep it at 11-12. After the reaction is complete, pour it into an autoclave for hydrothermal synthesis, set the temperature at 160°C, and hold the temperature for 1 hour. After washing, filtering, and drying to obtain hydroxyapatite nanoparticles; HA was made into a 0.01mol / L solution, and GD-DTPA was added with a weight ratio of HA:Gd-DTPA=10:1 under magnetic stirring (according to GD-DTPA DTPA injection concentration conversion), continue to stir for 12 hours, then wash, filter, and dry to obtain HA-Gd-DTPA particles, weigh 0.1gHA-Gd-DTPA and put it into 100ml water, add dispersant PEI (per gram of HA Add 0.001-0.05g) and ultrasonically disperse for 30min. The obtained solution is scann...
Embodiment 3
[0036] Weigh 0.1mol Ca(NO 3 ) 2 and 0.06mol(NH 4 ) 2 HPO 4 In a beaker, add 300ml of distilled water to form a solution and stir evenly. Use a dropper to dissolve (NH 4 ) 2 HPO 4 The solution was dropped into Ca(NO 3 ) 2 solution, use NH throughout the process 4 OH adjusts the pH to keep it at 11-12. After the reaction is complete, pour it into an autoclave for hydrothermal synthesis, set the temperature at 160°C, and hold the temperature for 1 hour. After washing, filtering, and drying, hydroxyapatite nanoparticles were obtained; HA was made into a 0.01mol / L solution, and GD-DTPA was added with a weight ratio of HA:Gd-DTPA=20:1 under magnetic stirring (according to GD-DTPA DTPA injection concentration conversion), continue to stir for 12 hours, then wash, filter, and dry to obtain HA-Gd-DTPA particles, weigh 0.1gHA-Gd-DTPA and put it into 100ml water, add PEI (0.001 -0.05g), and ultrasonically dispersed for 30min, the obtained solution was scanned under magnetic re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com