Drug compound for curing hyperlipoidemia and preparation method thereof
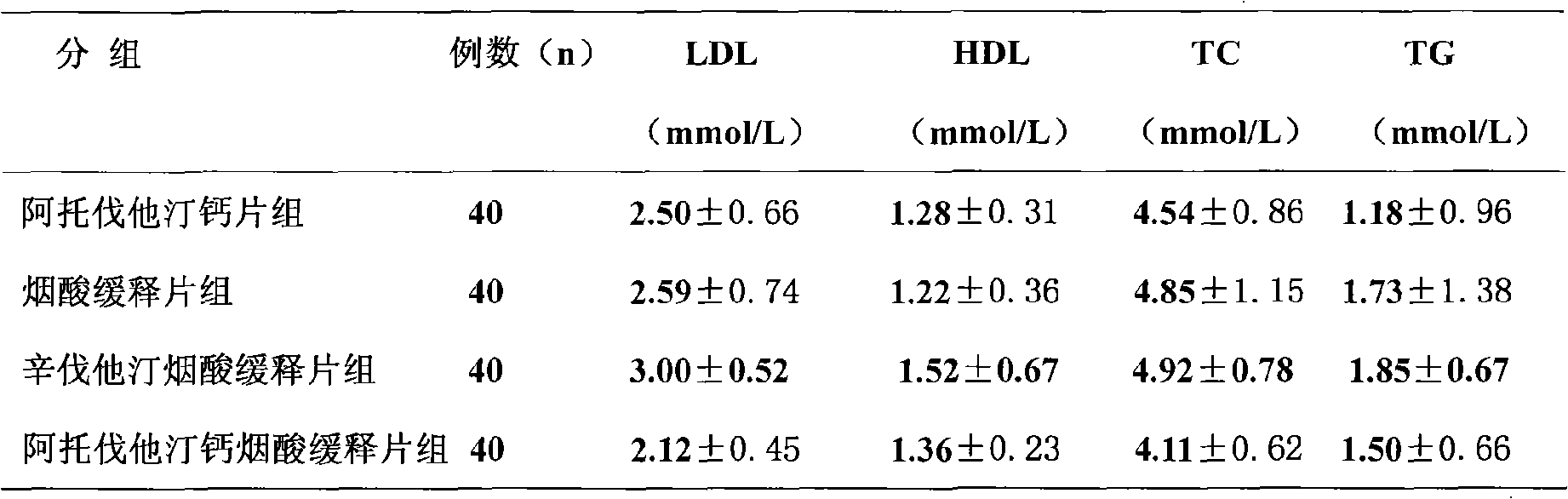
A technology of hyperlipidemia and composition, applied in the field of compound preparations, can solve the problems such as patent reports of atorvastatin and niacin sustained-release preparations that have not yet been found, and achieve the effects of reduced adverse reactions, reduced occurrence and convenient use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
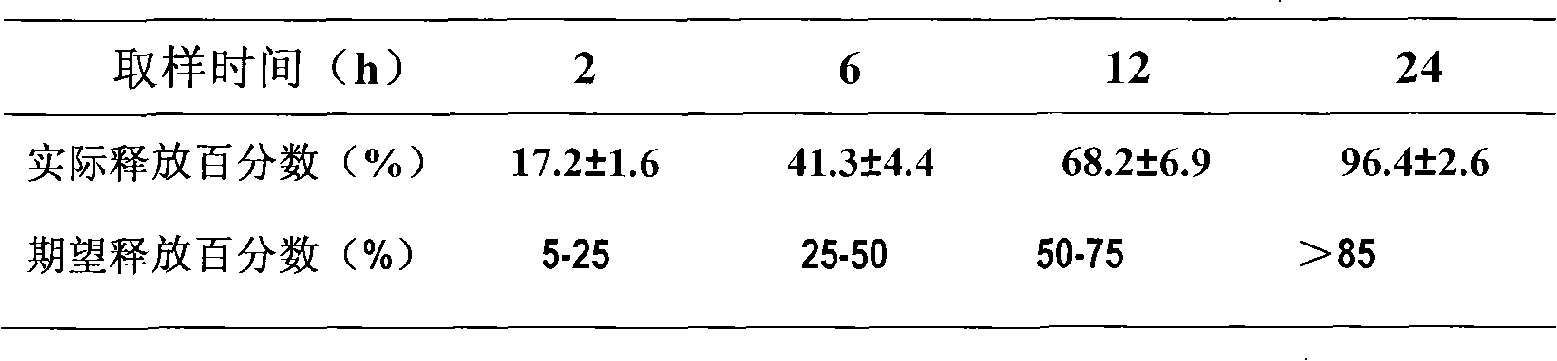
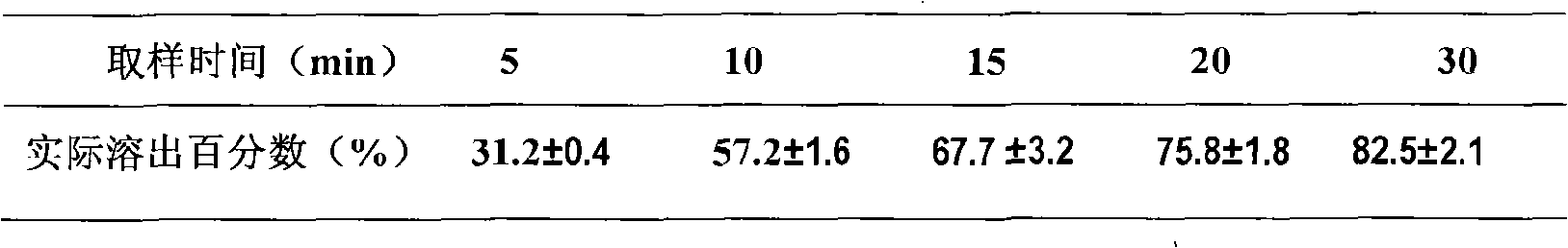
[0048] Embodiment 1 Atorvastatin Calcium Niacin Double Layer Sustained Release Tablet (20 / 500mg)
[0049] prescription:
[0050] Nicotinic acid layer
[0051] Component Dosage
[0052] Niacin 500.0g
[0053] HPMC-K15M 180.0g
[0054] Lactose 20.0g
[0056] Magnesium Stearate 3.5g
[0057] 5% starch slurry appropriate amount
[0058] Atorvastatin calcium layer
[0059] Component Dosage
[0060] Atorvastatin Calcium 20.0g
[0061] Microcrystalline Cellulose 100.0g
[0062] Lactose 40.0g
[0064] Magnesium Stearate 1.0g
[0065] 5% starch slurry appropriate amount
[0066] A total of 1000 pieces were made
[0067] Preparation:
[0068] Pass the prescribed amount of niacin through a 100-mesh sieve, and pass the prescribed amount of HPMC-K15M and lactose through a 60-mesh sieve. Mix the above raw and auxiliary materials evenly, make soft material with 5% starch slurry, granulate with 18 mesh sieve, dry at 50°C, g...
Embodiment 2
[0071] Example 2 Atorvastatin Calcium Niacin Double Layer Sustained Release Tablet (20 / 750mg)
[0072] prescription:
[0073] Nicotinic acid layer
[0074] Component Dosage
[0075] Niacin 750.0g
[0076] HPMC-K15M 230.0g
[0077] Lactose 30.0g
[0079] Magnesium Stearate 4.5g
[0080] 5% starch slurry appropriate amount
[0081] Atorvastatin calcium layer
[0082] Component Dosage
[0083] Atorvastatin Calcium 20.0g
[0084] Microcrystalline Cellulose 120.0g
[0085] Lactose 50.0g
[0086]Talc powder 2.0g
[0087] Magnesium Stearate 1.0g
[0088] 5% starch slurry appropriate amount
[0089] A total of 1000 pieces were made
[0090] Preparation:
[0091] Pass the prescribed amount of niacin through a 100-mesh sieve, and pass the prescribed amount of HPMC-K15M and lactose through a 60-mesh sieve. Mix the above raw and auxiliary materials evenly, make soft material with 5% starch slurry, granulate with 18 mesh sieve, dry at 50°C, granul...
Embodiment 3
[0094] Example 3 Atorvastatin Calcium Niacin Double Layer Sustained Release Tablet (20 / 1000mg)
[0095] prescription:
[0096] Nicotinic acid layer
[0097] Component Dosage
[0098] Niacin 1000.0g
[0099] HPMC-K100M 300.0g
[0100] Lactose 40.0g
[0101] Talc powder 15.0g
[0102] Magnesium Stearate 7.5g
[0103] 5% starch slurry appropriate amount
[0104] Atorvastatin calcium layer
[0105] Component Dosage
[0106] Atorvastatin Calcium 20.0g
[0107] Microcrystalline Cellulose 140.0g
[0108] Lactose 60.0g
[0109] Talc powder 2.0g
[0110] Magnesium Stearate 1.0g
[0111] 5% starch slurry appropriate amount
[0112] A total of 1000 pieces were made
[0113] Preparation:
[0114] Pass the prescribed amount of niacin through a 100-mesh sieve, and pass the prescribed amount of HPMC-K100M and lactose through a 60-mesh sieve. Mix the above raw and auxiliary materials evenly, make soft material with 5% starch slurry, granulate with 18 mesh sieve, dry at 50°C, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com