Method for preparing barium nitrate, calcium nitrate, calcium sulfate and sodium nitrate by utilizing middle and low grade barium carbonate ore
A low-grade, barium nitrate technology, applied in the preparation of calcium/strontium/barium nitrate, calcium/strontium/barium sulfate, alkali metal nitrate, etc., can solve the problems of environmental pollution, high production cost, and high cost of method 1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
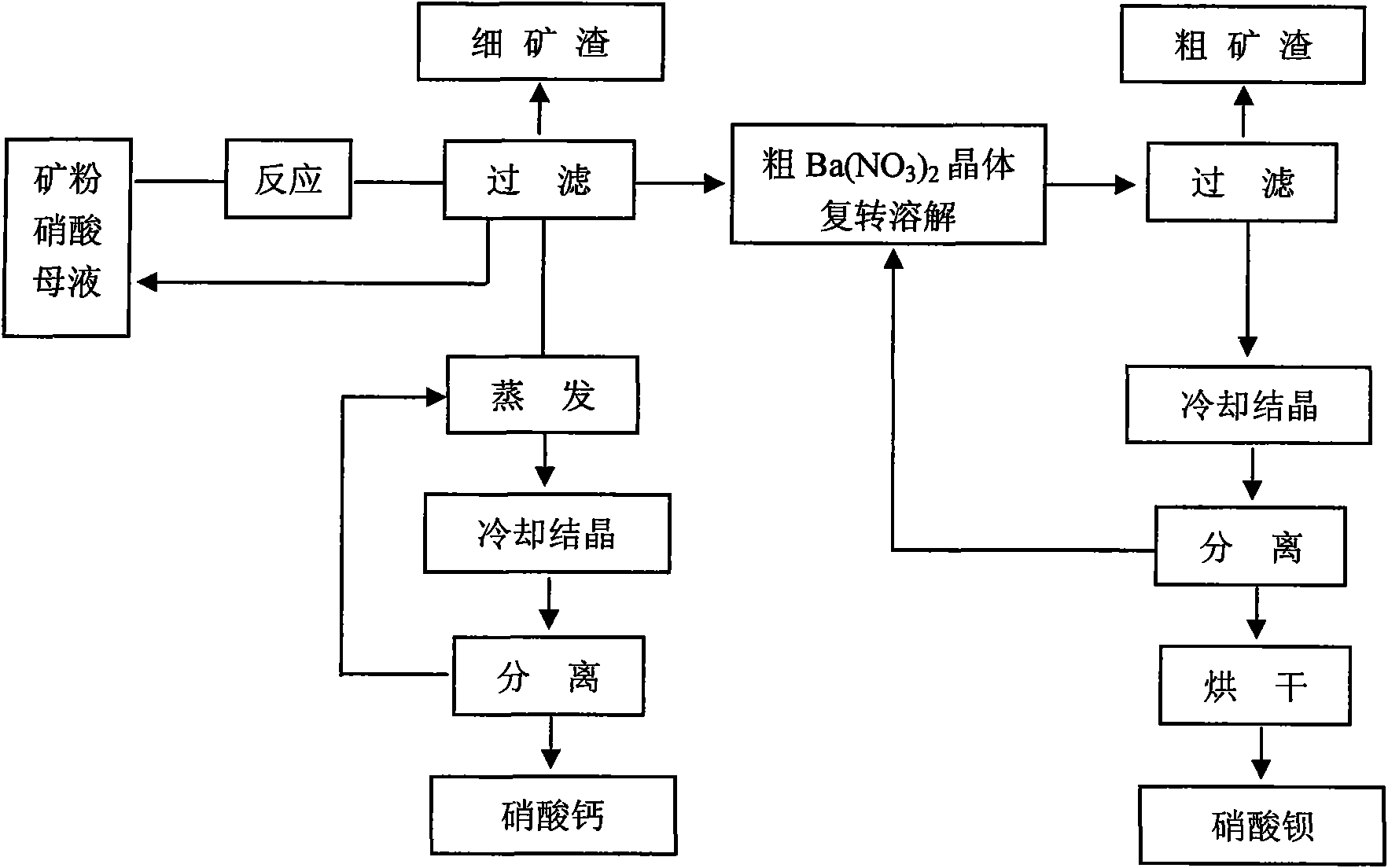
[0042] 1. Preparation of barium nitrate
[0043] 140g of circulating mother liquor with a concentration of 20.5wt% was added to a reaction kettle with a stirrer and a thermometer, and the mixture containing 35.5wt% BaCO was added under stirring. 3 and 58.3 wt% CaCO 3 50g parts by weight of medium and low-grade barium carbonate mineral powder, and 88g parts by weight of nitric acid with a concentration of 51.0wt%, were dissolved at room temperature for 15 minutes, heated to 60°C, stirred and reacted for 10 minutes, cooled to room temperature, and when pH=5.0, filtered to remove fine particles slag, and the crude product Ba(NO 3 ) 2 Crystal and filtrate 201g, crude product Ba(NO 3 ) 2 The crystal is redissolved, filtered to remove the coarse slag, the filtrate is cooled, crystallized, and separated: the solid is dried to obtain 30g of barium nitrate product, with a yield of 97% and a purity of 99.70wt%; the separated liquid is returned to the reconverted solution for use as ...
Embodiment 2
[0047] 1. Preparation of barium nitrate
[0048] 174.5g of circulating mother liquor with a concentration of 40wt% was added to a reaction kettle with a stirrer and a thermometer, and the mixture containing 66.5wt% BaCO was added under stirring. 3 and 18.4 wt% CaCO 3 50g of medium and low-grade barium carbonate ore powder, and 60g of nitric acid with a concentration of 51.0wt%, were dissolved at room temperature for 25 minutes, heated to 39°C, stirred and reacted for 110 minutes, cooled to room temperature, when pH=5.5, filtered to remove fine slag, and simultaneously obtained Crude Ba(NO 3 ) 2 Crystal and filtrate 202g, crude product Ba(NO 3 ) 2 The crystal is redissolved, filtered to remove the coarse slag, the filtrate is cooled, crystallized, and separated: the solid is dried to obtain 43.2g of barium nitrate product, the yield is 98%, and the purity reaches 99.8wt%; the separated liquid is returned to the reconverted solution use.
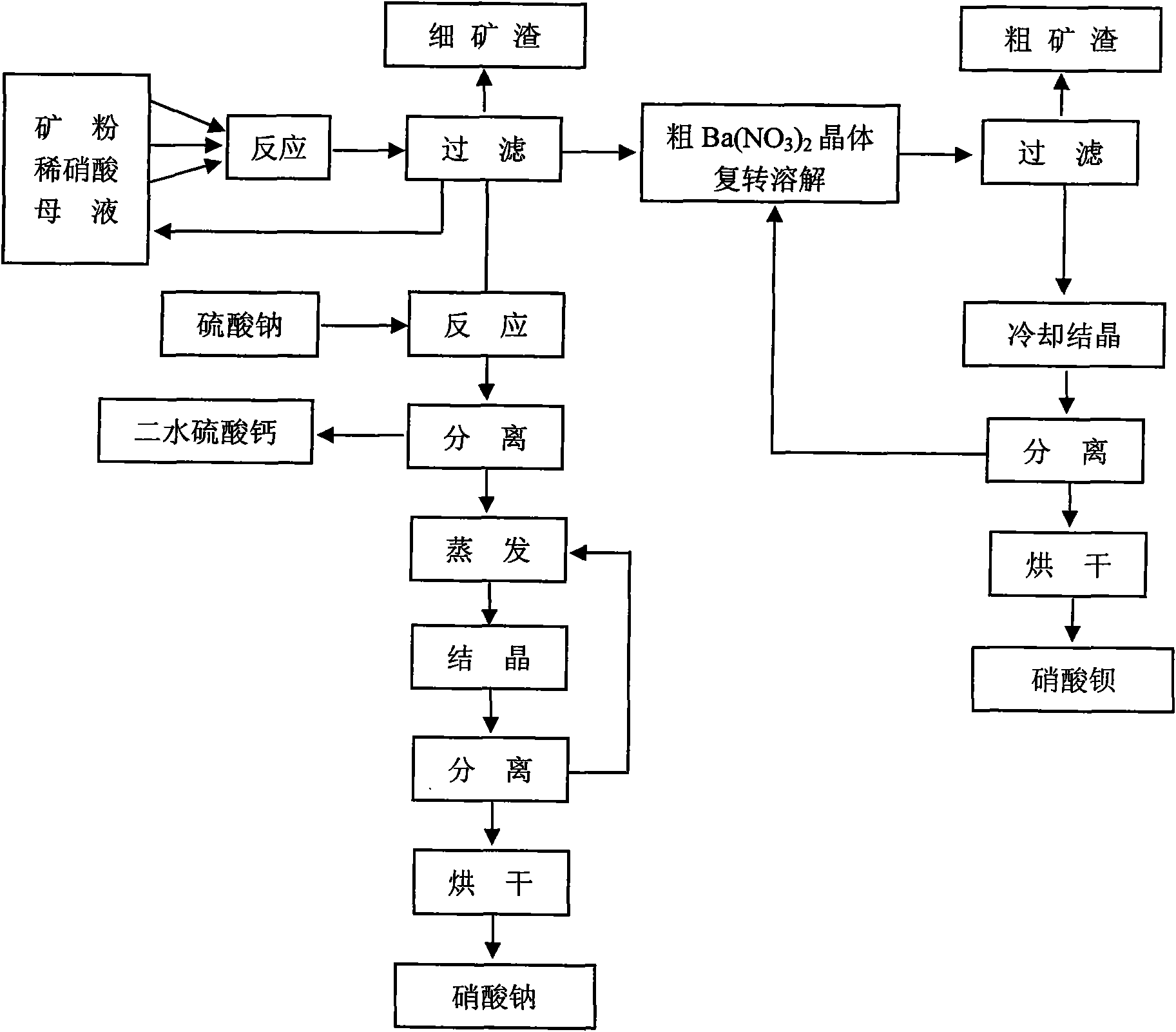
[0049] 2. Preparation of Calcium ...
Embodiment 3
[0052] 1. Preparation of barium nitrate
[0053] 160g of circulating mother liquor with a concentration of 38wt% was added to a reaction kettle with a stirrer and a thermometer, and the mixture containing 45.1wt% BaCO 3 and 39.8 wt% CaCO 3 50g of crushed ore powder of middle and low grade barium ore, and 86.5g of nitric acid with a concentration of 46.1wt%, were dissolved at room temperature for 25 minutes, heated to 50°C, stirred for 10 minutes, cooled to room temperature, and when pH = 6.0, filtered to remove fines slag, and the crude product Ba(NO 3 ) 2 Crystal and filtrate 199.8g, crude product Ba(NO 3 ) 2 The crystal is redissolved, filtered to remove the coarse slag, the filtrate is cooled, crystallized, and separated: the solid is dried to obtain 29.0 g of barium nitrate product by weight, with a yield of 98% and a purity of 99.78 wt%. Solvent used.
[0054] 2. Preparation of Calcium Sulfate
[0055] A part of 160g of the filtrate in step (1) is used as the next ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com