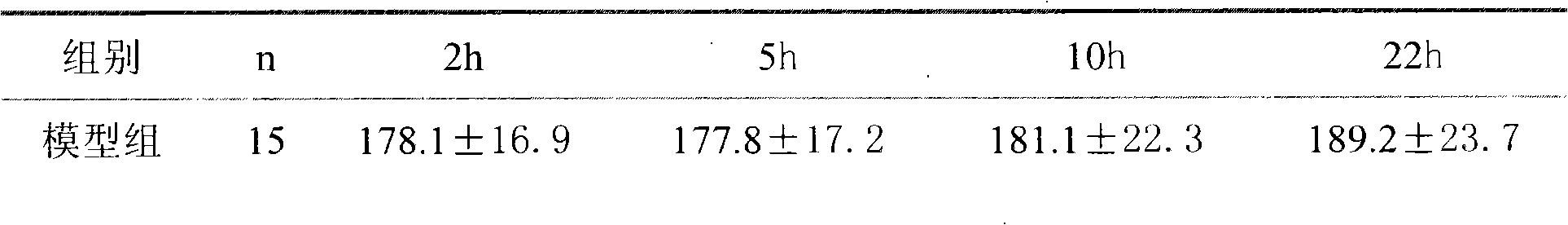Sustained and controlled release preparation for pharmaceutical composition for curing hypertension
A technology for controlled release preparations and high blood pressure, which is applied in the field of angiotensin II receptor blockers and sustained and controlled release preparations for the treatment of high blood pressure. The protection of structure and function, the reduction of drug side effects, and the effect of overcoming drug defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
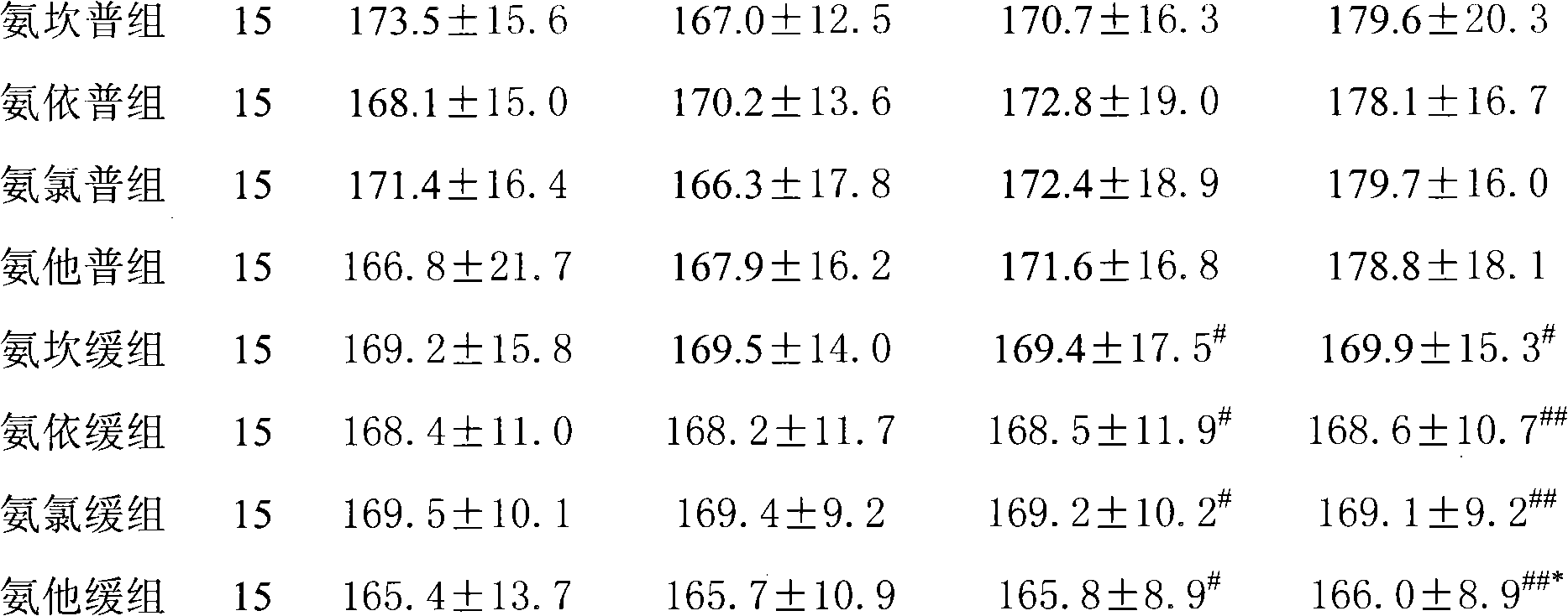
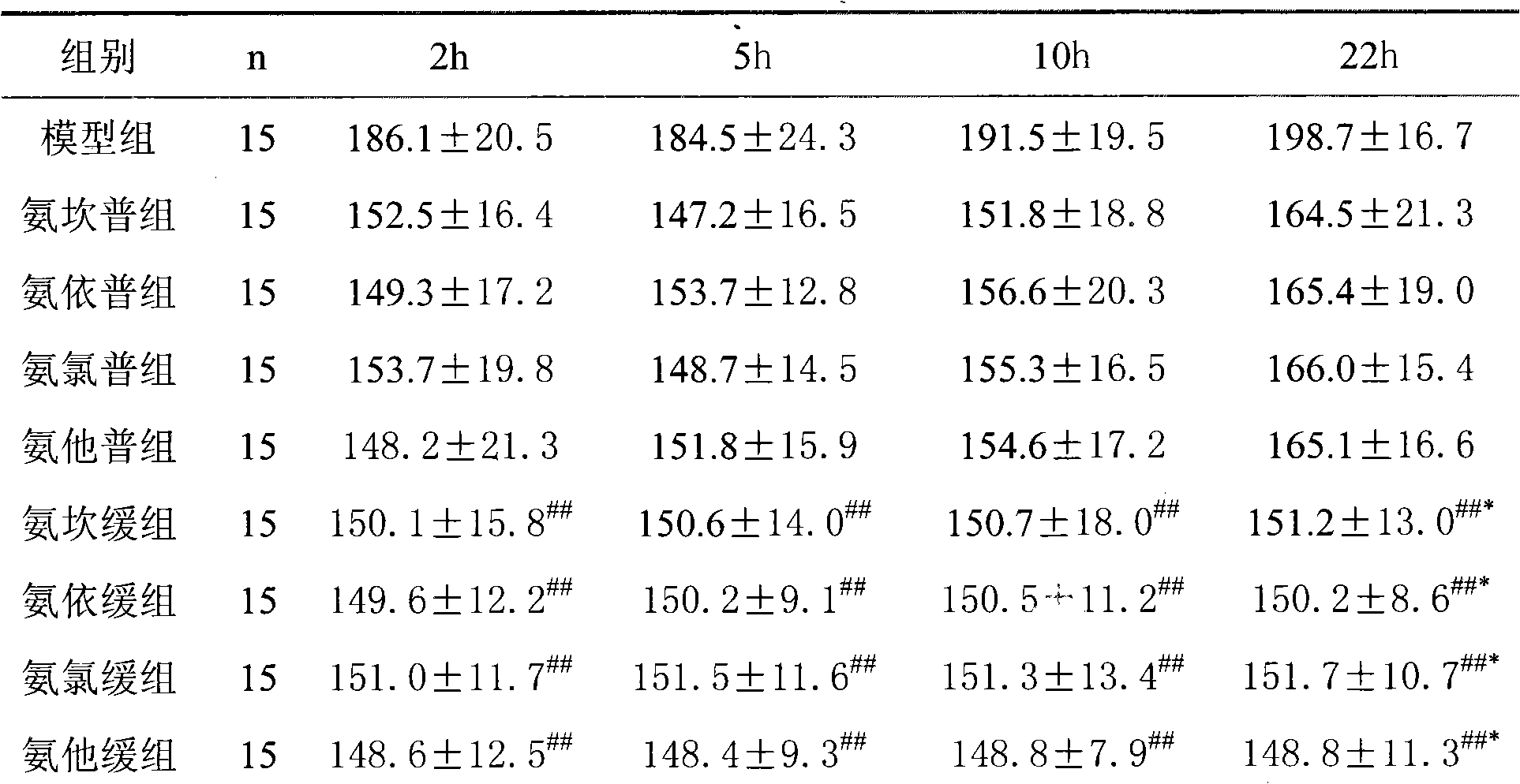
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 sustained release tablet
[0040] Sustained release part
[0041] Levoamlodipine Besylate 5g
[0042] Hydroxypropyl Methyl Cellulose 8g
[0043] Lactose 12g
[0044] 80% ethanol solution appropriate amount
[0045] common part
[0046] Candesartan cilexetil 2.5g
[0047] Microcrystalline Cellulose 15g
[0048] Dextrin 3g
[0049] 70% ethanol solution of 3% PVPk30 appropriate amount
[0050] Magnesium stearate 0.6g
[0051] Preparation Process:
[0052] First pass levamlodipine besylate and candesartan cilexetil through a 100-mesh sieve; hydroxypropyl methylcellulose, microcrystalline cellulose, lactose, and dextrin pass through an 80-mesh sieve.
[0053] Weigh levamlodipine besylate, hydroxypropyl methylcellulose, and lactose according to the prescription amount, mix them evenly, add 80% ethanol solution to make soft materials, pass through a 18-mesh sieve to granulate, and the wet granules are between 50 and 60 Dry at ℃, sieve...
Embodiment 2
[0055] The preparation of embodiment 2 sustained-release tablets
[0056] Sustained release part
[0057] Levoamlodipine Besylate 2.5g
[0058] Hydroxypropyl Methyl Cellulose 8g
[0059] Lactose 12g
[0060] 80% ethanol solution appropriate amount
[0061] common part
[0062] Candesartan cilexetil 4g
[0063] Microcrystalline Cellulose 15g
[0064] Starch 3g
[0065] 70% ethanol solution of 3% PVPk30 appropriate amount
[0066] Magnesium stearate 0.5g
[0067] Preparation Process:
[0068] First pass levamlodipine besylate and candesartan cilexetil through a 100-mesh sieve; hydroxypropyl methylcellulose, microcrystalline cellulose, lactose, and starch pass through an 80-mesh sieve.
[0069] Weigh levamlodipine besylate, hydroxypropyl methylcellulose, and lactose according to the prescription amount, mix them evenly, add 80% ethanol solution to make soft materials, pass through a 18-mesh sieve to granulate, and the wet granules are between 50 and 60 Dry at ℃, sieve th...
Embodiment 3
[0071] The preparation of embodiment 3 sustained-release tablets
[0072] Sustained release part
[0073] Levoamlodipine Besylate 5g
[0074] Hydroxypropyl Methyl Cellulose 750g
[0075] Lactose 120g
[0076] 80% ethanol solution appropriate amount
[0077] common part
[0078] Eprosartan Mesylate 600g
[0079] Microcrystalline Cellulose 450g
[0080] Starch 300g
[0081] 70% ethanol solution of 3% PVPk30 appropriate amount
[0083] Preparation Process:
[0084] First pass levamlodipine besylate and eprosartan mesylate through a 100-mesh sieve; hydroxypropyl methylcellulose, microcrystalline cellulose, lactose, and starch pass through an 80-mesh sieve.
[0085] Weigh levamlodipine besylate, hydroxypropylmethyl cellulose and lactose according to the prescription amount, mix the main drug levamlodipine besylate with the auxiliary materials by equal increment method, add 80% ethanol solution to make soft The material is granulated through...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com