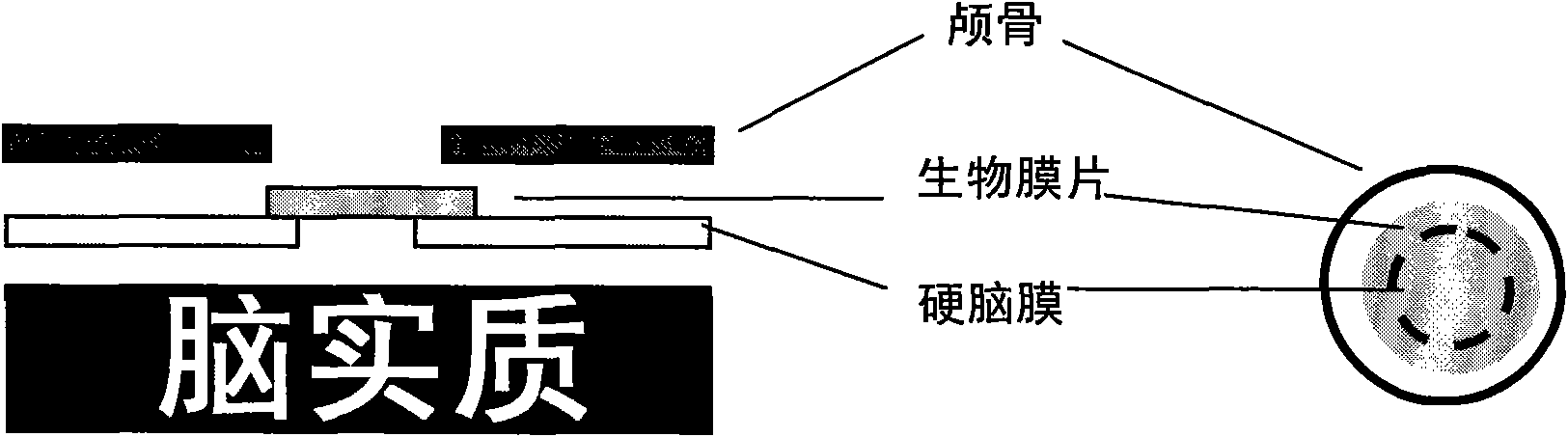
I-type medical collagen material keeping original specific triple helix structure of collagen, product and application thereof
A collagen material and triple helix technology, which is applied to type I medical collagen material and its products and application fields that maintain the unique triple helix structure of collagen, can solve the problems of scar tissue formation, influence of treatment effect, and unsatisfactory treatment effect. , to achieve the effect of reducing tissue adhesion, low immune antigenicity, and no foreign body reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Extraction of type I medical collagen materials that maintain the unique triple helix structure of collagen
[0025] Choose 1kg of bovine Achilles tendon tissue, freeze it, cut it into thin slices with a thickness of 0.1-3 mm, and immerse it in 0.1% sodium dihydrogen phosphate / sodium hydroxide solution for 1-3 hours under stirring conditions. Keep its pH neutral. Add 1:5 (w / w) ficin enzyme to the above-mentioned treated substance solution to fully react. With periodic stirring, thereafter treated with 1% ammonium nitrate for 6 hours. Then wash. Then add 1M sodium chloride for further treatment and control the temperature, adjust the pH, let stand overnight at 4°C, and then react with 2M equivalent sodium hydroxide solution. Then use 50% sulfuric acid to neutralize the buffer and adjust the pH to make it slightly acidic, take out the precipitate, wash and soak for 6 hours, then remove the water, and obtain the type I medical collagen material that maintains...
Embodiment 2
[0027] Embodiment 2: Preparation of meninges / spinal membranes biofilm
[0028] Take an appropriate amount of the type I medical collagen material with a triple helix structure in Example 1, dissolve 3.6 grams of collagen in 720 milliliters of 0.1M glacial acetic acid to make a 0.5% concentration of collagen suspension, and stir under high-speed and low-temperature conditions (20,000 rpm; 4°C) Add 6-chondroitin sulfate within 2 hours to obtain a 0.5% composite suspension, pour 720 ml into a 30×150 cm freezer tray, and put it in a freeze dryer freezer Freeze dried.
[0029] The setting of the freezing temperature is (1) -7°C / 30 minutes / in a continuous state; (2) -40°C / 200 minutes / in a curve state; (3) -40°C / 70 minutes / in a continuous state; freezing during the process The temperature of the dryer freezer was maintained at -80°C. The temperature of the sublimation stage was set to (1) 0°C / 18 hours; (2) the temperature of the second heating was set to 20°C for 40 minutes, and th...
Embodiment 3
[0032] Embodiment 3: Preparation of meninges / spinal membrane biofilm
[0033] Take an appropriate amount of the type I medical collagen material with a triple helix structure of Example 1, dissolve 7.2 grams in 0.1M glacial acetic acid of 720 milliliters, and make a collagen suspension with a concentration of 2%, under high-speed low-temperature stirring conditions ( 30,000 rpm; 0°C) Add 6-chondroitin sulfate within 1.5 hours to obtain a 1% compound suspension, take 125 ml and pour it into a 12.5×12.5 cm freezer tray, and put it in a freeze dryer freezer for freezing dry.
[0034] The setting of the freezing temperature is (1) -9°C / 30 minutes / in a continuous state; (2) -50°C / 220 minutes / in a curve state; (3) -45°C / 80 minutes / in a continuous state; freezing during the process The temperature of the dryer freezer was maintained at -80°C. The temperature in the sublimation stage was set to (1) 0°C / keep for 20 hours; (2) the temperature for the second heating was set to 20°C for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com