Curcumin-4-nitrogen-containing derivatives, and preparation method and uses thereof
A technology of curcumin and its derivatives, which is applied in the fields of antibacterial multifunctional dyes and photosensitive materials, and curcumin-4-nitrogen-containing derivatives, which can solve the problems of curcumin's poor water solubility, easy oxidation, and low curcumin absorption rate, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of Compound 1
[0029] Dissolve 3mmol curcumin in 20mL tetrahydrofuran, after cooling to 0~5 ℃, dropwise add 16.5% (weight percent) 7mmol sodium nitrite aqueous solution 3mL, 37% (weight percent) concentrated hydrochloric acid 1mL successively, then in ice-water bath The reaction was stirred for 2 to 6 hours, and the reaction was detected by TLC. After the reaction was completed, most of the solvent was distilled off under reduced pressure, 30 mL of water was added to the residue, and the pH was adjusted to 6 to 7 with 10% (weight percent) aqueous sodium bicarbonate solution. Suction filtration, washing with water, and vacuum drying to obtain the crude product, which was separated and purified by silica gel column chromatography (eluent: methanol: chloroform = 1: 10-8, volume ratio) to obtain compound 1 (purity over 98%).
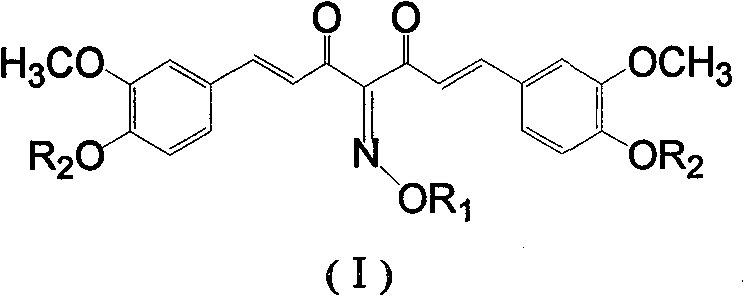
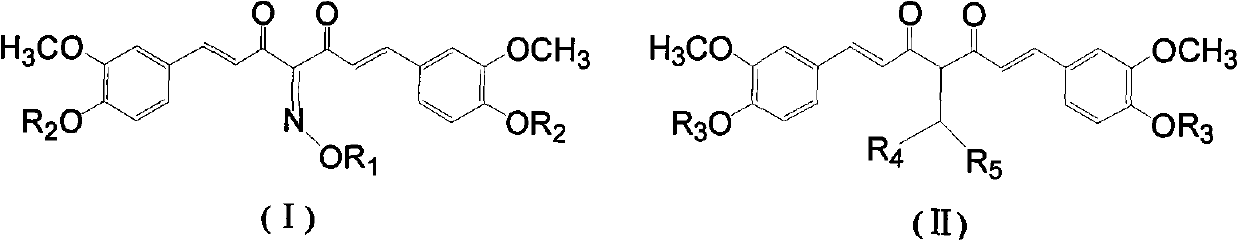
[0030] Compound 11,7-bis(4-hydroxy-3-methoxy)phenyl-4-hydroxyimino-1,6-heptadiene-3,5-dione (common name: curcumin-4-oxime)
...
Embodiment 2
[0033] Example 2 Preparation of Compound 2
[0034] Get 3mmol two-O-acetyl curcumin and be dissolved in 30mL tetrahydrofuran, after cooling to 0~5 ℃, drip 7mmol sodium nitrite aqueous solution 3mL of 16.5% (weight percent) successively, and 37% (weight percent) concentrated 1 mL of hydrochloric acid was then stirred in an ice-water bath for 2 to 8 hours, and the reaction was detected by TLC. After the reaction was completed, most of the solvent was distilled off under reduced pressure, 30 mL of water was added to the residue, and 10% (weight percent) sodium bicarbonate was used. The pH of the aqueous solution was adjusted to 6-7, filtered with suction, washed with water, and dried in vacuo to obtain the crude product, which was separated and purified by silica gel column chromatography (eluent: methanol:chloroform=1:10-8, volume ratio) to obtain compound 2 ( more than 98% purity).
[0035] Compound 2 1,7-bis(4-acetoxy-3-methoxy)phenyl-4-hydroxyimino-1,6-heptadiene-3,5-dione (...
Embodiment 3
[0038] Example 3 Preparation of compound 3
[0039] Add 2.4 mmol of piperidine to 20 mL of tetrahydrofuran (THF), add concentrated hydrochloric acid dropwise in an ice-water bath to adjust the pH to 6, dropwise add 2.4 mmol of formaldehyde, introduce a stream of nitrogen, and slowly add 20 mL of 20 mL of curcumin dissolved in 0 to 5°C dropwise. The tetrahydrofuran solution was continuously stirred for 4-10 h. The reaction was detected by TLC, the solvent was recovered after the reaction, and the 10% (weight percent) aqueous sodium bicarbonate solution was neutralized to pH 6~7, and the crude product was isolated and obtained, which was subjected to silica gel column chromatography (eluent: methanol: chloroform=1: 12~8 , volume ratio) separation and purification to obtain compound 3 (purity above 98%).
[0040] Compound 3 1,7-bis(4-hydroxy-3-methoxy)phenyl-4-(N-piperidinylmethyl)-1,6-heptadiene-3,5-dione
[0041]
[0042] Khaki powder (43.8% yield), mp 185-187°C; IR (KBr) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com