Quinoline compound, pharmaceutical composition, preparation method and application thereof
A technology of compounds and quinolines, applied in the field of pharmaceutical chemical synthesis, can solve the problem of insufficient efficacy of blood lipid-lowering drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
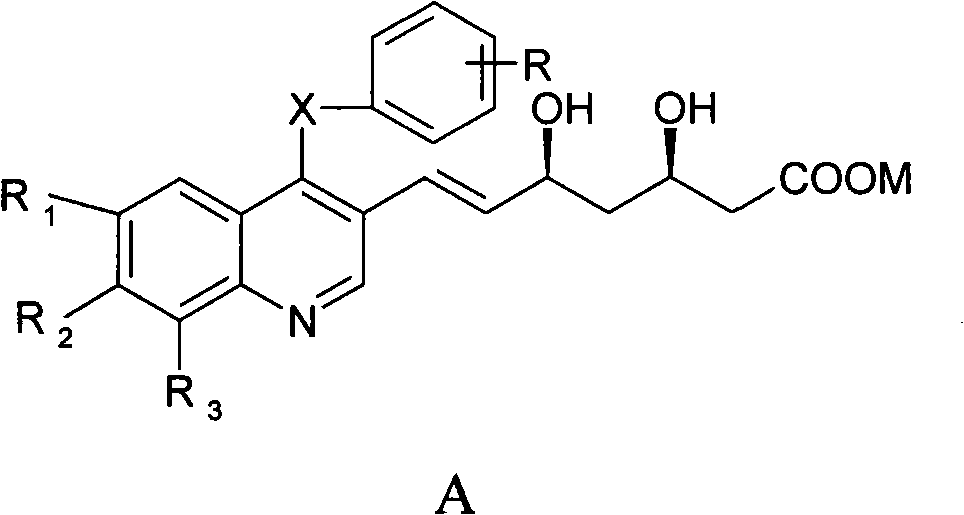
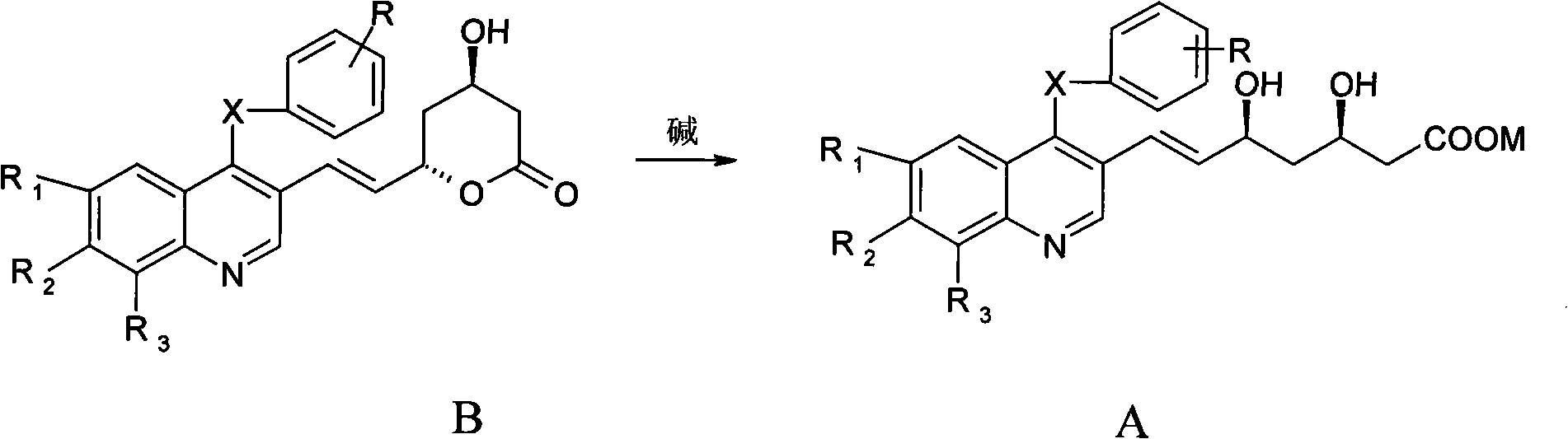
[0046] Example 1: (3R,5S)-7-[4,6,7,8-tetra-(p-isopropylphenylthio)quinolin-3-yl]-3,5-dihydroxy-6( E)-Heptenoic acid (A1)
[0047] (4R,6S)-6-[(E)-2-(4,6,7,8-tetra-p-isopropylphenylthioquinoline-3-)vinyl]-3,4,5, 6-Tetrahydro-4-hydroxy-2H-pyran-2-one 0.5g (0.58mmol) and 5ml THF (tetrahydrofuran) were cooled to 0℃, 1N NaOH 0.8ml (0.8mmol) was added, and stirred for 1h at 0℃ Adjust the pH value to 2 with 1N HCl, concentrate the reaction solution under reduced pressure, add water and ethyl acetate, separate the organic layer, extract the aqueous layer three times with ethyl acetate, combine the organic layers, wash with water until neutral, anhydrous Na 2 SO 4 Dry and concentrate to obtain 0.4 g of solid, yield 78%, Mp (melting point): 118-120°C, [ α ] D 26 = 18.2 (c 1, THF). 1 H NMR(400MHz, DMSO-d 6 )δ1.17-1.11 (m, 18H), 1.25 (d, 6H, J=6.8 Hz), 1.62-1.53 (m, 2H)...
Embodiment 2
[0048] Example 2: (3R,5S)-7-[6-fluoro-7-chloro-4-(m-methoxyphenylthio)quinolin-3-yl]-3,5-dihydroxy-6(E )-Heptenoic acid (A2)
[0049] (4R,6S)-6-[(E)-2-(6-fluoro-7-chloro-4-m-methoxyphenylthioquinolin-3-yl)vinyl]-3,4,5,6 -Tetrahydro-4-hydroxy-2H-pyran-2-one 0.27g (0.58mmol) and 5ml methanol to cool to 10℃, add 1N KOH 0.8ml (0.8mmol), stir for 5h, adjust pH with 1NHCl at 25℃ When the value reached 2, the reaction solution was concentrated under reduced pressure, water and ethyl acetate were added, the organic layer was separated, the aqueous layer was extracted three times with ethyl acetate, the organic layers were combined, washed with water until neutral, anhydrous Na 2 SO 4 Dry and concentrate to obtain 0.26 g of solid, yield 94.7%, Mp: 178-180°C, [ α ] D 26 = 31.8 (c 1, methanol). 1 H NMR(400MHz, DMSO-d 6 )δ1.69-1.59(m, 2H), 2.42-2.25(m, 2H), 3.67(s, 3H), ...
Embodiment 3
[0050] Example 3: (3R,5S)-7-[6-fluoro-4,7-di-m-methoxyphenylthioquinolin-3-yl]-3,5-dihydroxy-6(E)- Heptenoic acid (A3)
[0051] 25℃, 1N NaOH 0.8ml (0.8mmol) was added (4R, 6S)-6-[(E)-2-(6-fluoro-4,7-di-methoxyphenylthioquinolin-3-yl )Vinyl]-3,4,5,6-tetrahydro-4-hydroxy-2H-pyran-2-one 0.33g (0.58mmol) and 5ml acetone solution, stir for 8h, use 1N HCl at 0℃ Adjust the pH to 2, concentrate the reaction solution under reduced pressure, add water and ethyl acetate, separate the organic layer, extract the aqueous layer three times with ethyl acetate, combine the organic layers, wash with water until neutral, anhydrous Na 2 SO 4 Dry and concentrate to obtain 0.29 g of solid, yield 85.3%, Mp: 154-156°C, [ α ] D 26 = 21.6 (c 1, methanol). 1 H NMR(400MHz, DMSO-d 6 )δ1.68-1.58(m, 2H), 2.5-2.24(m, 2H), 3.67(s, 3H), 3.78(s, 3H), 4.01(brs, 1H), 4.36(brs, 1H), 4.69 (brs, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com