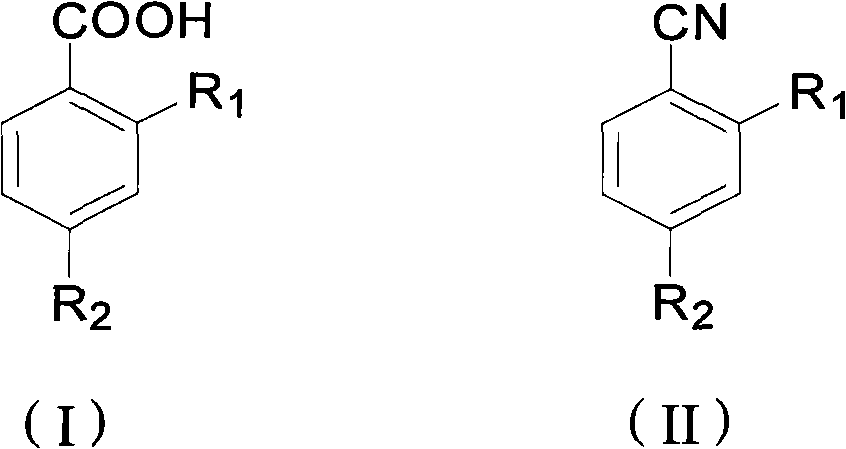
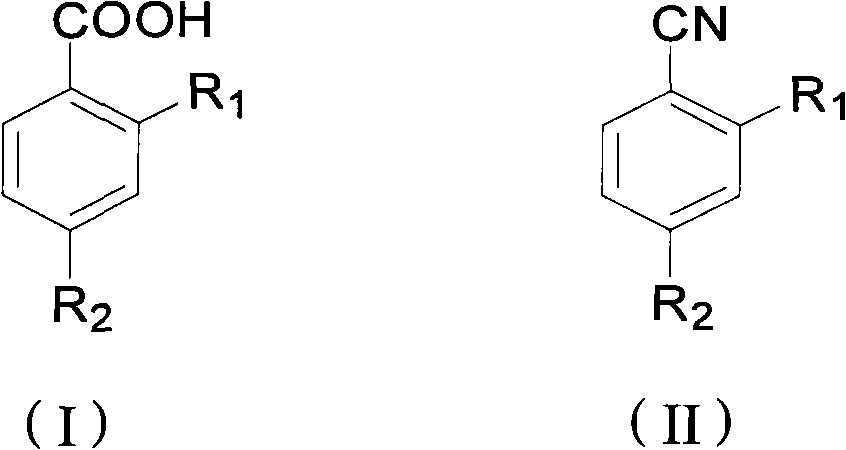
Preparation method of ortho/para-substituted phenylformic acid compound
A technology of benzoic acids and compounds, which is applied in the field of preparation of benzoic acid compounds, can solve problems such as low reaction yield and difficult product separation, and achieve the effects of good purity, shortened reaction time, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Preparation of 2-methylthio-4-trifluoromethylbenzoic acid
[0021] Add 4.4g (0.02mol) of 2-methylthio-4-trifluorobenzonitrile, 3.2g (0.08mol) of sodium hydroxide, 2mL of water, and 40mL of ethylene glycol to the reaction flask in sequence, start stirring, and heat to 140 ℃, reacted for 3 hours, and took samples for HPLC chromatographic detection and analysis. The reaction conversion rate was 99.6%, and the reaction selectivity was 98.3%. Cool down to 30°C, add 30%% hydrochloric acid to adjust the pH to 6, filter, wash the filter cake with 50mL of water, dry, weigh to obtain 4.4g, and the yield is 93.6%. 1 H NMR (500NMR, d 6 -acetone): δ 2.54 (s, 3H), 7.54 (q, 1H), 7.63 (s, 1H), 8.19 (d, 1H).
Embodiment 2
[0022] Example 2 Preparation of 2-methylthio-4-trifluoromethylbenzoic acid
[0023] Add 26.1g (0.12mol) of 2-methylthio-4-trifluorobenzonitrile, 27g (0.48mol) of potassium hydroxide, 10mL of water, and 100mL of glycerin to the reaction flask in sequence, start stirring, and heat to a temperature of 140°C , reacted for 3 hours, sampled for HPLC chromatographic detection and analysis, the reaction conversion rate was 97.3%, and the reaction selectivity was 99%. Cool down to 30°C, add 30% hydrochloric acid to adjust the pH value of the reaction solution to 6, filter, wash the filter cake with 200mL water, dry, weigh 27.2g, and the product yield is 96.1%.
Embodiment 3
[0024] Example 3 Preparation of 2-nitro-4-trifluoromethylbenzoic acid
[0025] Add 8.7g (0.04mol) of 2-nitro-4-trifluorobenzonitrile, 16g (0.28mol) of potassium hydroxide, 10mL of water, and 50mL of ethylene glycol in the reaction flask, start stirring, and heat to a temperature of 140 ℃, reacted for 4 hours, sampled for HPLC chromatographic detection and analysis, the reaction conversion rate was 98.6%, and the reaction selectivity was 99.2%. Cool down to 30°C, add 30% hydrochloric acid to adjust the pH of the reaction solution to 6, filter, wash the filter cake with 100 mL of water, dry, weigh to obtain 8.9 g, and the product yield is 94.7%. 1 H NMR (500NMR, CDCl 3 ): δ8.02(d, 1H), 8.07(q, 1H), 8.26(s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com