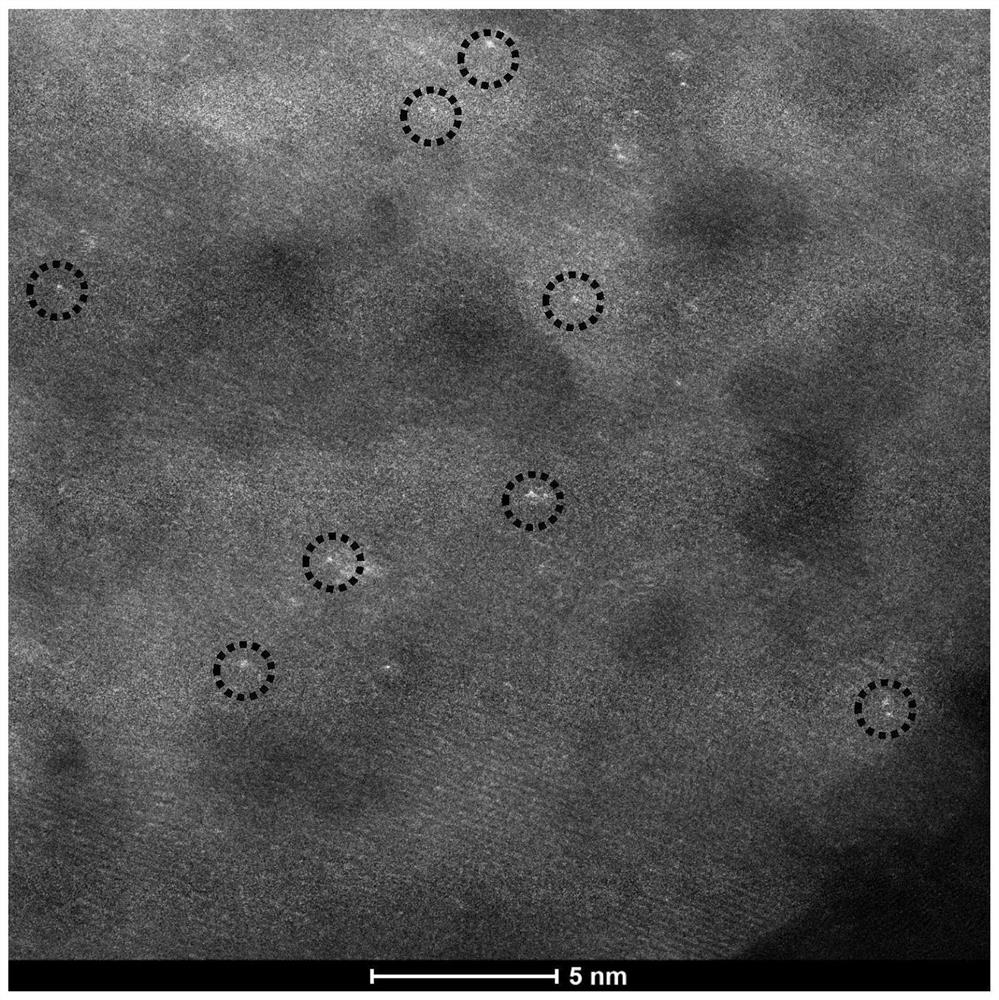
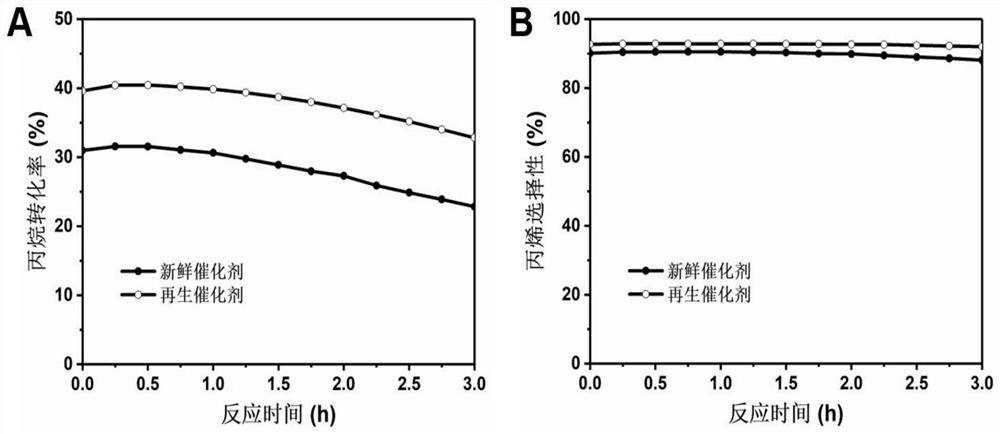
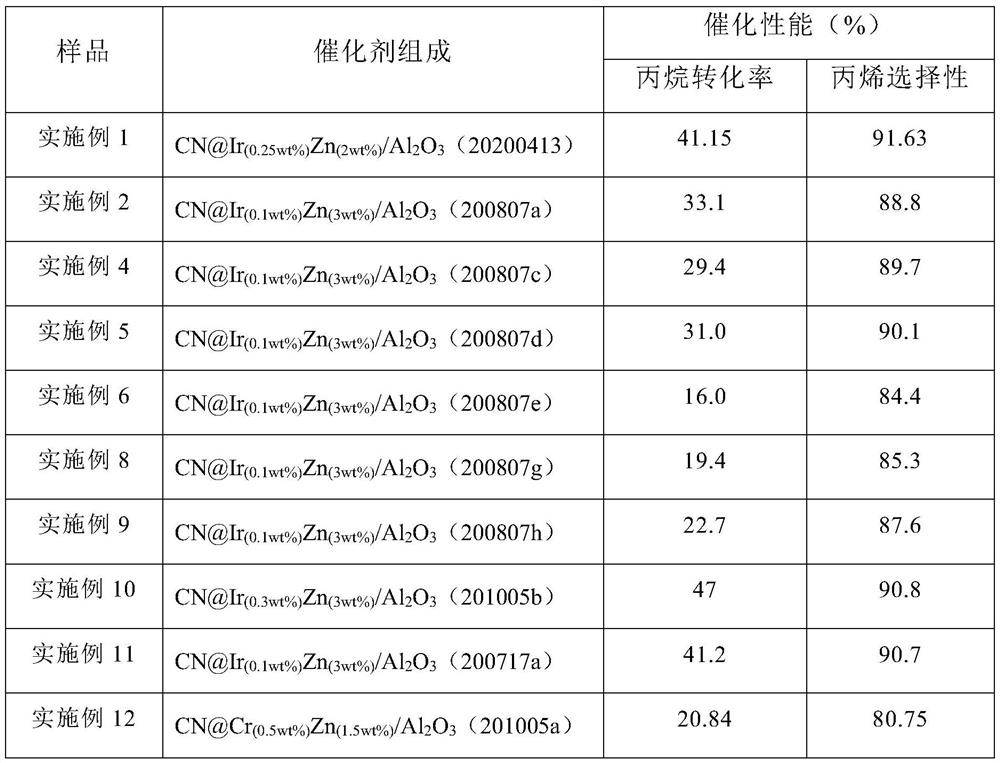
Preparation method and application of CN (at) Ma-Mb supported monatomic catalyst
A catalyst and atomic technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic chemistry, etc., can solve problems such as high toxicity of chromium compounds, carbon deposition in catalysts, and damage to NC carriers, so as to improve conversion rate and selectivity sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1, CN@Ir (0.25wt%) Zn (2wt%) / Al 2 O 3 (20200413)
[0063] Take 6.7g of iridium chloride trihydrate and 6.7g of sodium chloride with water to dissolve and dilute to 1200g, then add 167.8g of zinc nitrate hexahydrate to dissolve, add 1Kg of small spherical Al 2 O 3 , soaked for 0.5 hours, drained, and dried at 60°C for 24 hours. Iridium-zinc-supported Al can be prepared 2 O 3 Catalyst, labeled Ir (0.25wt%) Zn (2wt%) / Al 2 O 3 .
[0064] The iridium-zinc catalyst was coated using 2-methylimidazole (2-MI) as the CN precursor. 287.7g of 2-methylimidazole was dissolved in 1200g of water in advance, and the above Ir (0.25wt%) Zn (2wt%) / Al 2 O 3 After soaking for 0.5 hours, take out, drain, dry at 120 °C for 3 hours, and then dry at 600 °C, N 2 The CN-coated iridium-zinc catalyst can be prepared by pyrolysis under protective atmosphere for 3 hours, which is marked as CN@Ir (0.25wt%) Zn (2wt%) / Al 2 O 3 .
Embodiment 2
[0065] Example 2, CN@Ir (0.1wt%) Zn (3wt%) / Al 2 O 3 (200807a)
[0066] Prepare an aqueous solution of Ir with a concentration of 0.005g / g with iridium chloride and sodium chloride in advance, take 20.0g, add 13.6g of zinc nitrate hexahydrate, and use H 2 O was diluted to 110.0 g, followed by the addition of 96.9 g of small spherical Al 2 O 3 , followed by rotary evaporation, until the solvent is completely evaporated and the iridium and zinc species are fully loaded on the Al 2 O 3surface, iridium-zinc-supported Al can be prepared 2 O 3 Catalyst, labeled Ir (0.1wt%) Zn (3wt%) / Al 2 O 3 .
[0067] The iridium-zinc catalyst was coated with 2-methylimidazole (2-MI) as the CN precursor, and the molar ratio of 2-methylimidazole to Zn was 2:1. A 2-methylimidazole aqueous solution with a concentration of 7.53 wt% was pre-configured, 5.0 g was taken, and 5.0 g of Ir was added. (0.1wt%) Zn (3wt%) / Al 2 O 3 After soaking for 0.5 hours, take it out, absorb the remainin...
Embodiment 3
[0068] Example 3, CN@Ir (0.1wt%) Zn (3wt%) / Al 2 O 3 (200807b)
[0069] Ir (0.1wt%) Zn (3wt%) / Al 2 O 3 For the preparation method, see the relevant steps in Example 2. The iridium-zinc catalyst was coated with 2-methylimidazole (2-MI) as the CN precursor, and the molar ratio of 2-methylimidazole to Zn was 3:1. Pre-configured 2-methylimidazole aqueous solution with a concentration of 11.3wt%, take 5.0g, add 5.0g of Ir (0.1wt%) Zn (3wt%) / Al 2 O 3 After soaking for 0.5 hours, take it out, absorb the remaining liquid with paper towels, dry at 120 °C for 3 hours, and then heat it at 600 °C under N 2 The CN-coated iridium-zinc catalyst can be prepared by pyrolysis under protective atmosphere for 3 hours, which is marked as CN@Ir (0.1wt%) Zn (3wt%) / Al 2 O 3 (MI-3).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com