Technological method for preparing benzaldehyde through methyl benzoate hydrogenation
A technology of methyl benzoate and a process method, which is applied in the field of chemical engineering, can solve problems such as increased hydrogen recycling operating costs, increased separation costs, and difficulty in condensation, and achieves suppression of side reactions, improvement of conversion rate and selectivity, and process methods Simple and easy-to-operate effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 10 g of the catalyst was loaded into a tubular reactor with an inner diameter of 20 mm. The catalyst was purged with hydrogen and nitrogen gas mixture (volume ratio 1:1) at 400°C for 0.5h, and the space velocity of the purge gas was 100mL / min.
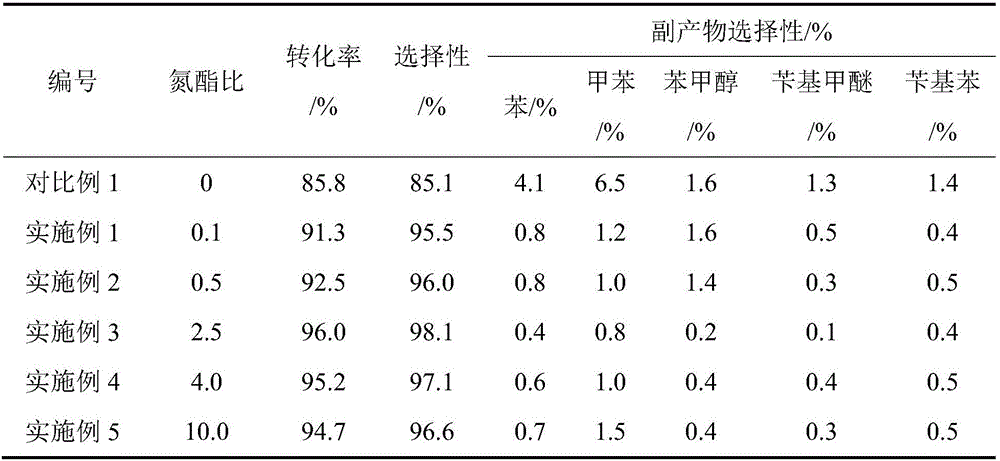
[0021] After that, methyl benzoate was treated with 0.25h -1 Space Velocity Liquid Phase Feed. When the hydrogen ester molar ratio is 2.5, the hydrogen gas flow rate is 17.14 mL / min; when the nitrogen ester molar ratio is 0.1, the nitrogen gas flow rate is 0.69 mL / min. Under the conditions of temperature 430°C and pressure 0.4MPa, the reaction of hydrogenation to benzaldehyde is carried out. The conversion rate of methyl benzoate was 91.3%, and the selectivity of forming benzaldehyde was 95.5%.
Embodiment 2
[0023] 10 g of the catalyst was loaded into a tubular reactor with an inner diameter of 20 mm. The catalyst was purged with hydrogen and nitrogen gas mixture (volume ratio 1:1) at 400°C for 0.5h, and the space velocity of the purge gas was 100mL / min.
[0024] After that, methyl benzoate was treated with 0.25h -1 Space Velocity Liquid Phase Feed. When the hydrogen ester molar ratio is 2.5, the hydrogen gas flow rate is 17.14 mL / min; when the nitrogen ester molar ratio is 0.5, the nitrogen gas flow rate is 3.43 mL / min. Under the conditions of temperature 430°C and pressure 0.4MPa, the reaction of hydrogenation to benzaldehyde is carried out. The conversion rate of methyl benzoate was 92.50%, and the selectivity of forming benzaldehyde was 96.0%.
Embodiment 3
[0026] 10 g of the catalyst was loaded into a tubular reactor with an inner diameter of 20 mm. The catalyst was purged with hydrogen and nitrogen gas mixture (volume ratio 1:1) at 400°C for 0.5h, and the space velocity of the purge gas was 100mL / min.
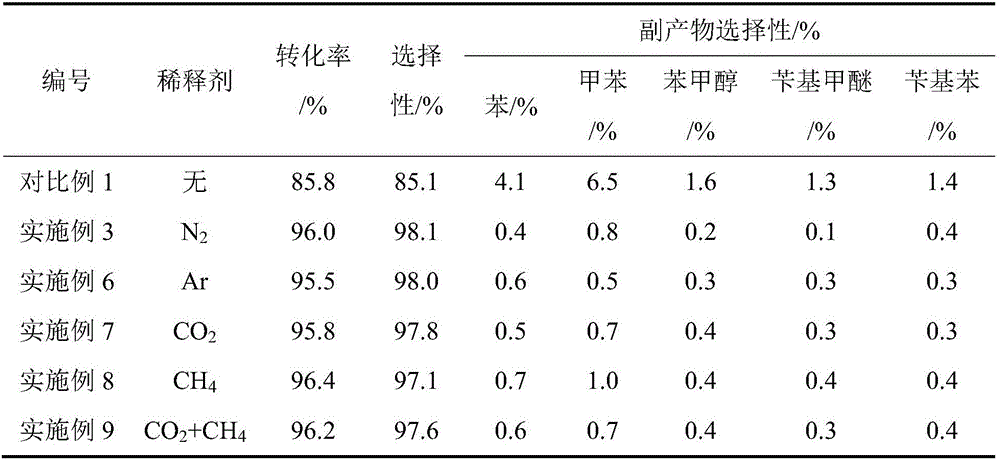
[0027] After that, methyl benzoate was treated with 0.25h -1 Space Velocity Liquid Phase Feed. The hydrogen ester molar ratio is 2.5, the hydrogen gas flow rate is 17.14mL / min; the nitrogen ester molar ratio is 2.5, the nitrogen gas flow rate is 17.14mL / min. Under the conditions of temperature 430°C and pressure 0.4MPa, the reaction of hydrogenation to benzaldehyde is carried out. The conversion rate of methyl benzoate was 96.0%, and the selectivity of forming benzaldehyde was 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com