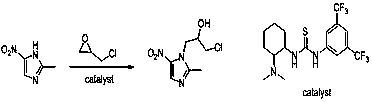
Process for preparing 5-nitroimidazole medicine through catalysis of small organic molecules
A technology of nitroimidazoles and small molecule catalysis, which is applied in the fields of organic chemistry, organic compound/hydride/coordination complex catalyst, chemical/physical process, etc. The problem of large amount of three wastes is achieved to solve the effect of large equipment corrosion, high conversion rate and selectivity, and small usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
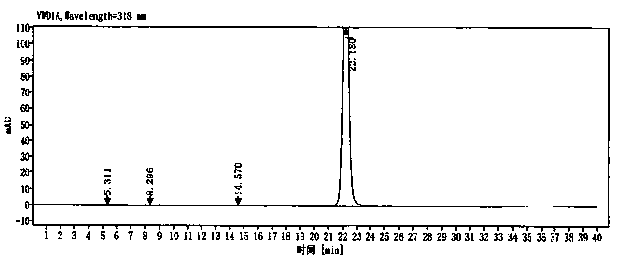
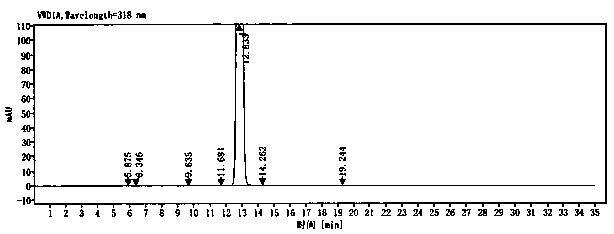
Image
Examples
Embodiment 1
[0055] Add 127.1 g of 2-methyl-5-nitroimidazole, 635.5 g of ethyl acetate, and 4.13 g of catalyst to the reaction flask. Stir and mix evenly. The stirring speed is 50~300rpm.
[0056] Add 127.1 g of epichlorohydrin to the above reaction flask,
[0057] Control the temperature in the reaction flask to 25~30℃, stir the reaction for 6 hours, and stir at 50~300rpm;
[0058] After the reaction is over, add 1 times the weight of 2-methyl-5-nitroimidazole (127.1g) of water, adjust the pH to about 4.0 with 1 mol / L hydrochloric acid, stand still and separate to obtain an organic phase and water phase;
[0059] Adjust the pH value of the aqueous phase to about 8.0 with 1.25 mol / L sodium hydroxide solution, and solids will precipitate out. After suction filtration, the solid phase is rinsed with a small amount of water and dried to obtain the catalyst, which can be directly applied.
[0060] The organic phase is washed with water 1 times the weight of 2-methyl-5-nitroimidazole, separated into lay...
Embodiment 2
[0064] Add 127.1 g of 2-methyl-5-nitroimidazole, 1271.0 g of butyl acetate, and 6.3 g of catalyst to the reaction flask. Stir and mix evenly. The stirring speed is 50~300rpm.
[0065] Add 139.0 g of epichlorohydrin to the above reaction flask,
[0066] Control the temperature in the reaction flask at 20~35℃, stir the reaction for 8 hours, and stir at 50~300rpm;
[0067] After the reaction is over, add water (127.1g) that is 1 times the weight of 2-methyl-5-nitroimidazole, adjust the pH to about 5.5 with 2 mol / L hydrochloric acid, stand still and separate to obtain the water phase and organic phase;
[0068] The pH of the aqueous phase is adjusted to about 10 with 2.5 mol / L sodium hydroxide solution, and solids are separated out, filtered with suction, the solids are rinsed with a small amount of water, dried to obtain the catalyst, which can be directly applied.
[0069] The organic phase was washed with water 1 times the weight of 2-methyl-5-nitroimidazole (127.1g), separated into lay...
Embodiment 3
[0072] Add 127.1 g of 2-methyl-5-nitroimidazole, 635.5 g of ethyl acetate, and 4.13 g of catalyst to the reaction flask, stir and mix well, at a stirring speed of 50~300 rpm,
[0073] Add 115.6 g of propylene oxide to the above reaction flask,
[0074] Control the temperature in the reaction flask to 25~30℃, stir the reaction for 6 hours, and stir at 50~300rpm;
[0075] After the reaction is over, add 1 times the weight of 2-methyl-5-nitroimidazole (127.1g) of water, adjust the pH to about 4 with 1 mol / L hydrochloric acid, stand still and separate to obtain the organic phase and water phase;
[0076] The pH of the aqueous phase is adjusted to about 8 with 1.25 mol / L sodium hydroxide solution, and solids are precipitated. After suction filtration, the solids are rinsed with a small amount of water and dried to obtain the catalyst, which can be used directly.
[0077] The organic phase was washed with water 1 times the weight of 2-methyl-5-nitroimidazole (127.1g), separated into layers, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com