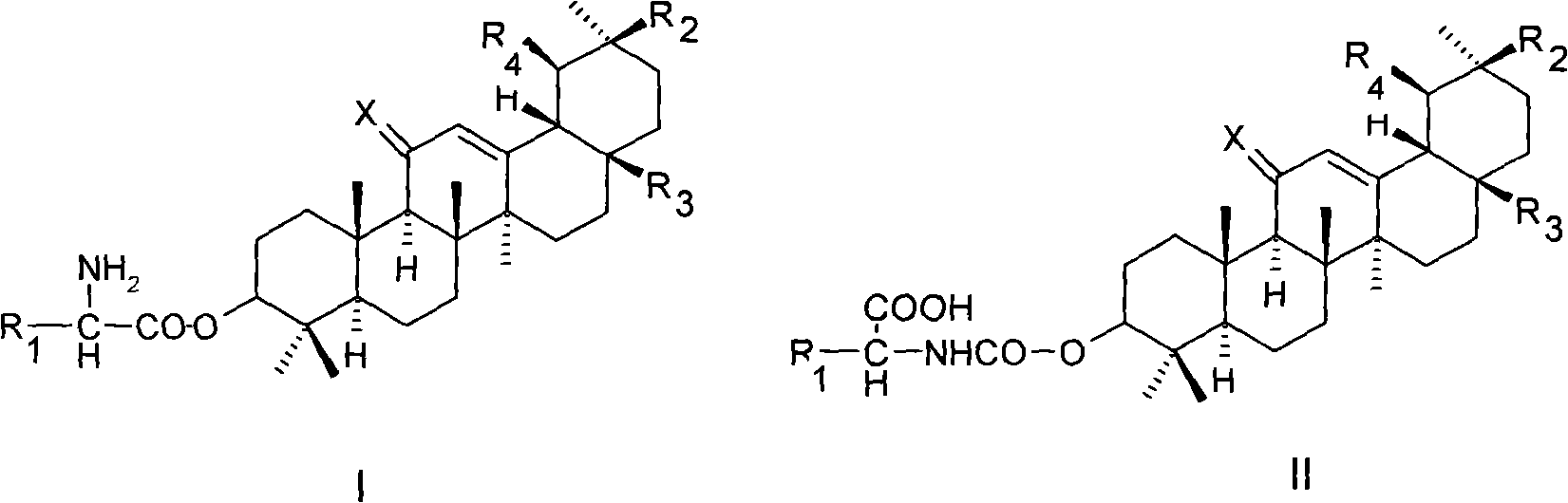Amino acid conjugate prodrug of pentacyclic triterpenoid and medical application thereof
A technology of pentacyclic triterpenoids and conjugates, which is applied in the field of prodrugs of amino acid conjugates of pentacyclic triterpenoids and their medical applications, and can solve the problems that oral preparations are difficult to exert curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
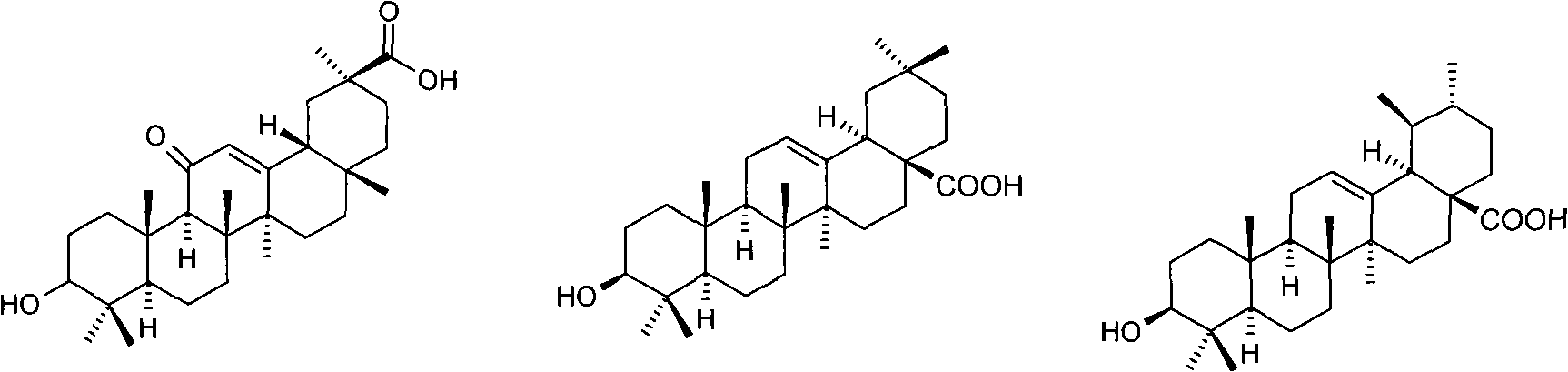
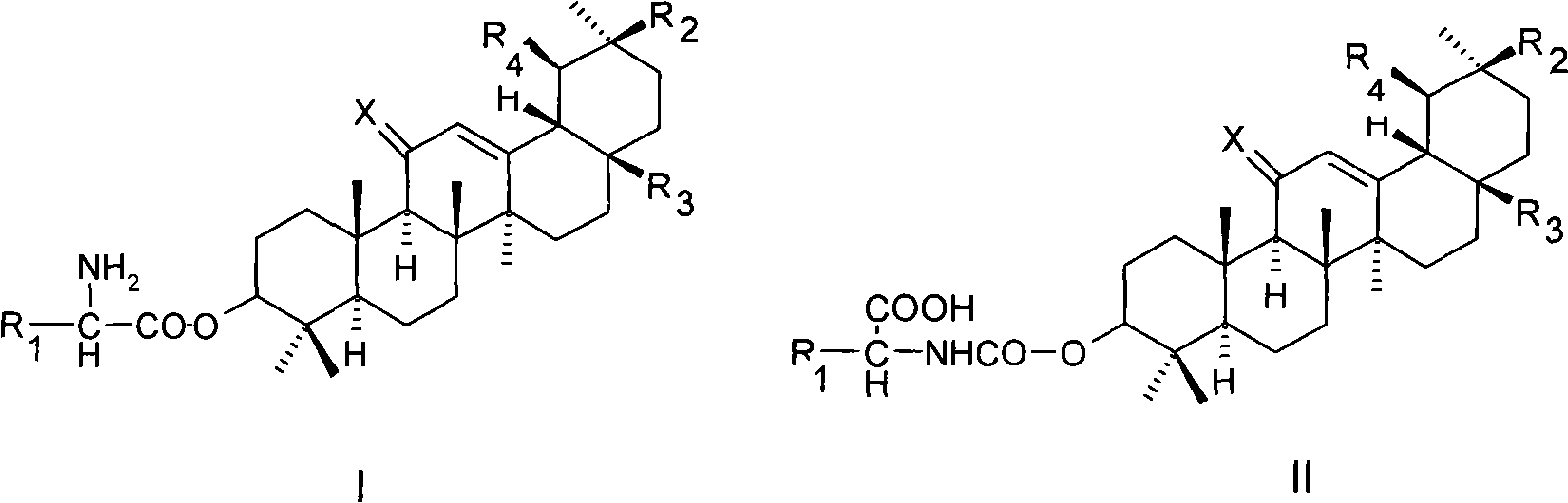
[0020] Example 1 3-O-(glycyl)-oleanolic acid (I 1 ) preparation
[0021] Dissolve 2.3g (5.0mmol) oleanolic acid, 1.1g (5.0mmol) N-benzyloxycarbonylglycine with 25ml dimethylformamide, then add 0.1ml dimethylaminopyridine, 1.3g (6mmol) dicyclohexyl Carbodiimide, stirred overnight at room temperature, filtered, and evaporated the filtrate to dryness under reduced pressure. The residue was separated by silica gel column chromatography, eluted with a mixed solvent of dichloromethane / methanol / acetic acid (9:1:0.01), and the desired components were collected and evaporated to dryness under reduced pressure to obtain 3-O-(N-benzyl Oxycarbonylglycyl)-oleanolic acid 2.4 g.
[0022] Dissolve 2.4 g of 3-O-(N-benzyloxycarbonylglycyl)-oleanolic acid in 25 ml of dioxane, add 0.2 g of 5% palladium carbon, and stir for 4 hours under 1 atmosphere of hydrogen Afterwards, the catalyst was filtered off. The filtrate was concentrated under reduced pressure, then separated by silica gel column ...
Embodiment 2
[0024] Example 2 3-O-(L-alanyl)-oleanolic acid (I 2 ) preparation
[0025] According to the method of Example 1, replace N-benzyloxycarbonylglycine with N-benzyloxycarbonyl-L-alanine, and condense with oleanolic acid under the action of dicyclohexylcarbodiimide to obtain 3-O-( N-Benzyloxycarbonyl-L-alanyl)-oleanolic acid.
[0026] According to the method of Example 1, replace 3-O-(N-benzyloxycarbonylglycyl)-oleanolic acid with 3-O-(N-benzyloxycarbonyl-L-alanyl)-oleanolic acid, Catalytic hydrogenation deprotection affords I 2 . Proton NMR spectrum: δ(ppm, DMSO-d 6 ):0.72(S,3H);0.82(S,3H);0.87(S,3H);0.90(S,3H);0.91(S,3H);0.95(S,3H);1.11(S,3H) ;1.26(d,3H);2.76(br d,1H);3.54(q,1H);4.78(dd,1H);5.16(br s,1H);8.27(br s,2H); , 1H).
Embodiment 3
[0027] Example 3 3-O-(L-valyl)-oleanolic acid (I 3 ) preparation
[0028] According to the method of Example 1, replace N-benzyloxycarbonylglycine with N-benzyloxycarbonyl-L-valine, and condense with oleanolic acid under the action of dicyclohexylcarbodiimide to obtain 3-O- (N-Benzyloxycarbonyl-L-valyl)-oleanolic acid.
[0029] Using 3-O-(N-benzyloxycarbonyl-L-valyl)-oleanolic acid instead of 3-O-(N-benzyloxycarbonylglycyl)-oleanolic acid, catalytic hydrogenation deprotection, to obtain I 3 . Proton NMR spectrum: δ(ppm, DMSO-d 6 ):0.73(S,3H);0.82(S,3H);0.87(S,3H);0.90(S,3H);0.91(S,3H);0.92(m,6H);0.96(S,3H) ; 1.11(S, 3H); 2.25(m, 1H); 2.75(br d, 1H); 3.69(m, 1H); 4.76(dd, 1H); 2H); 12.20 (br S, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com