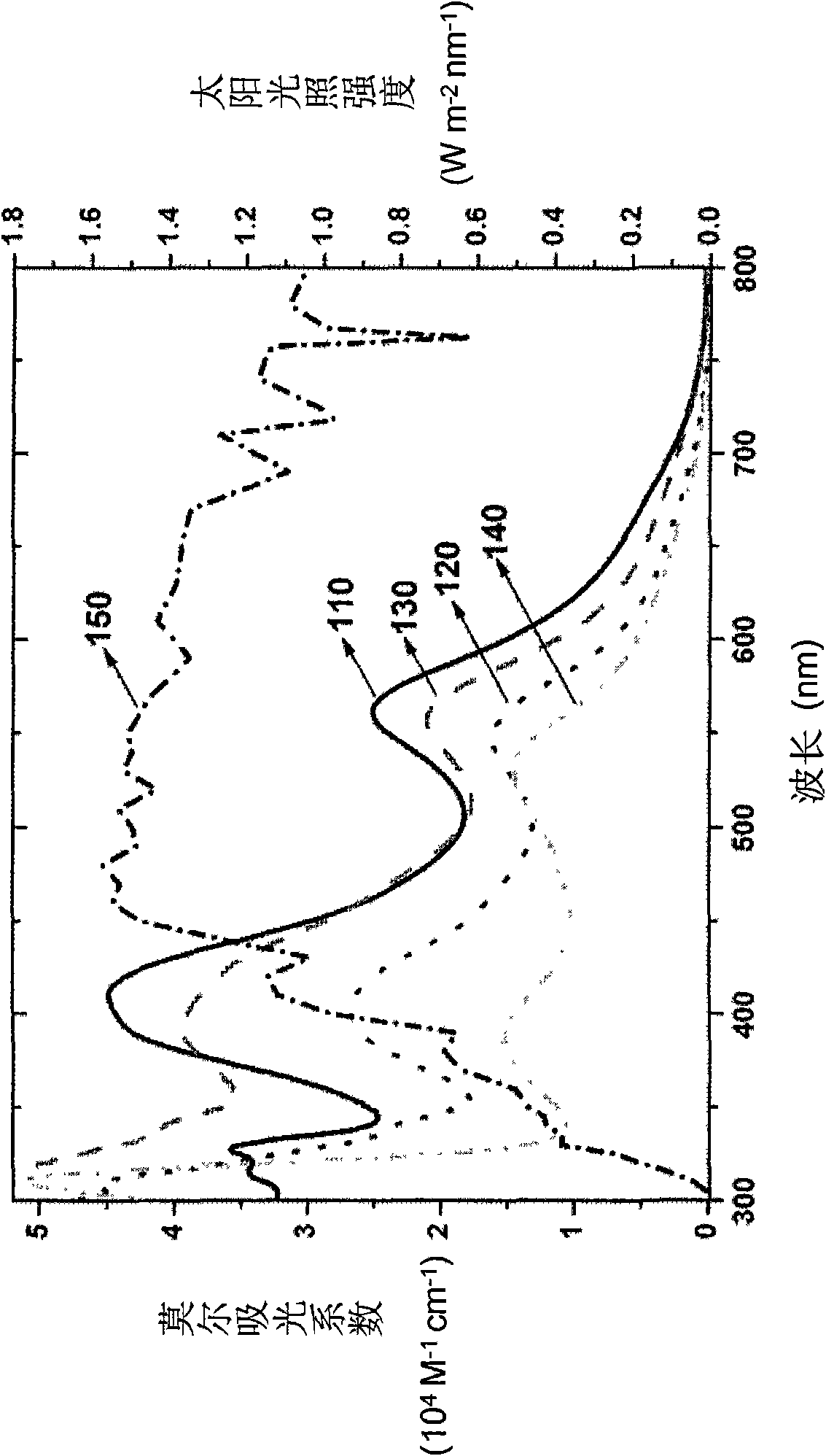
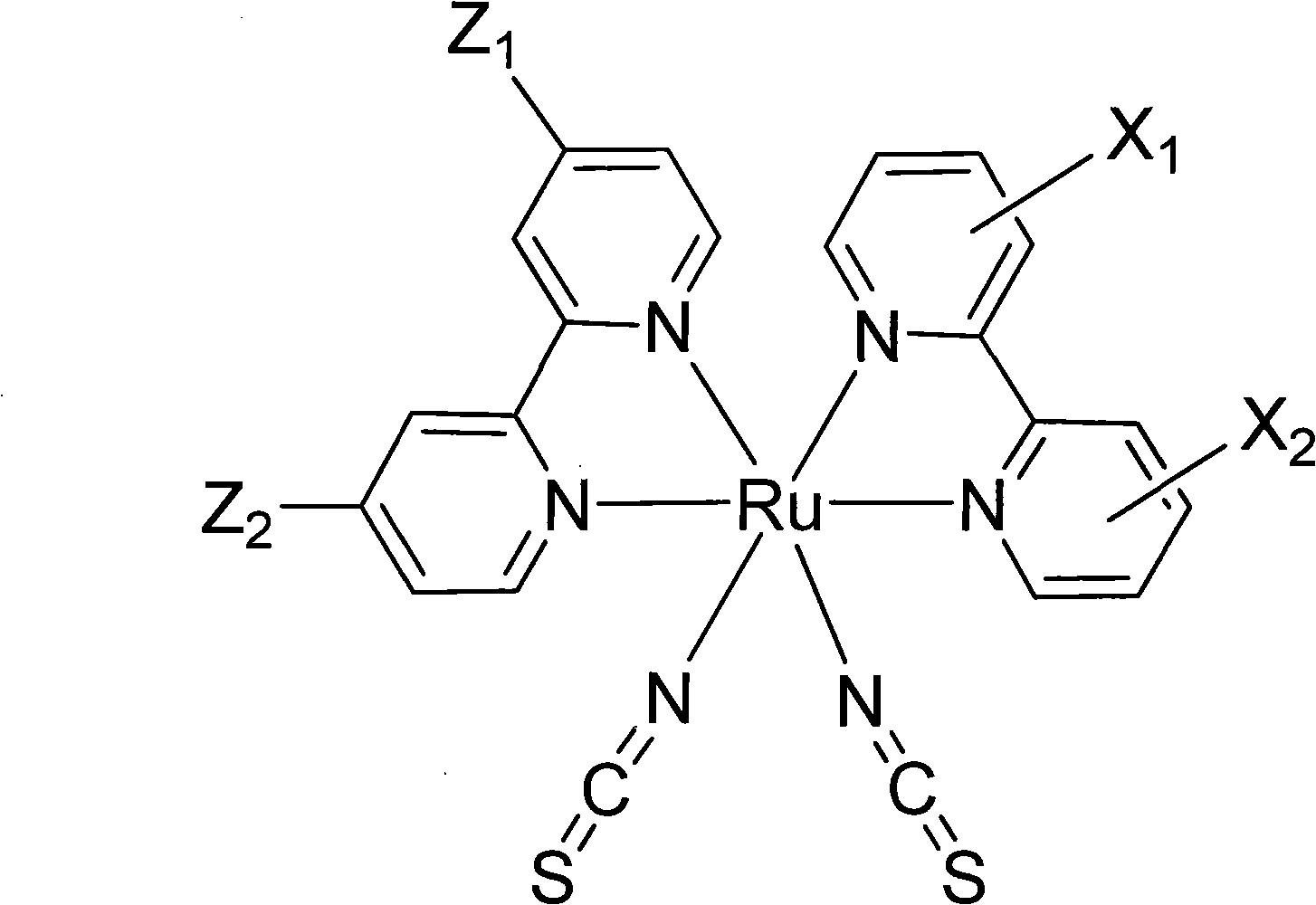
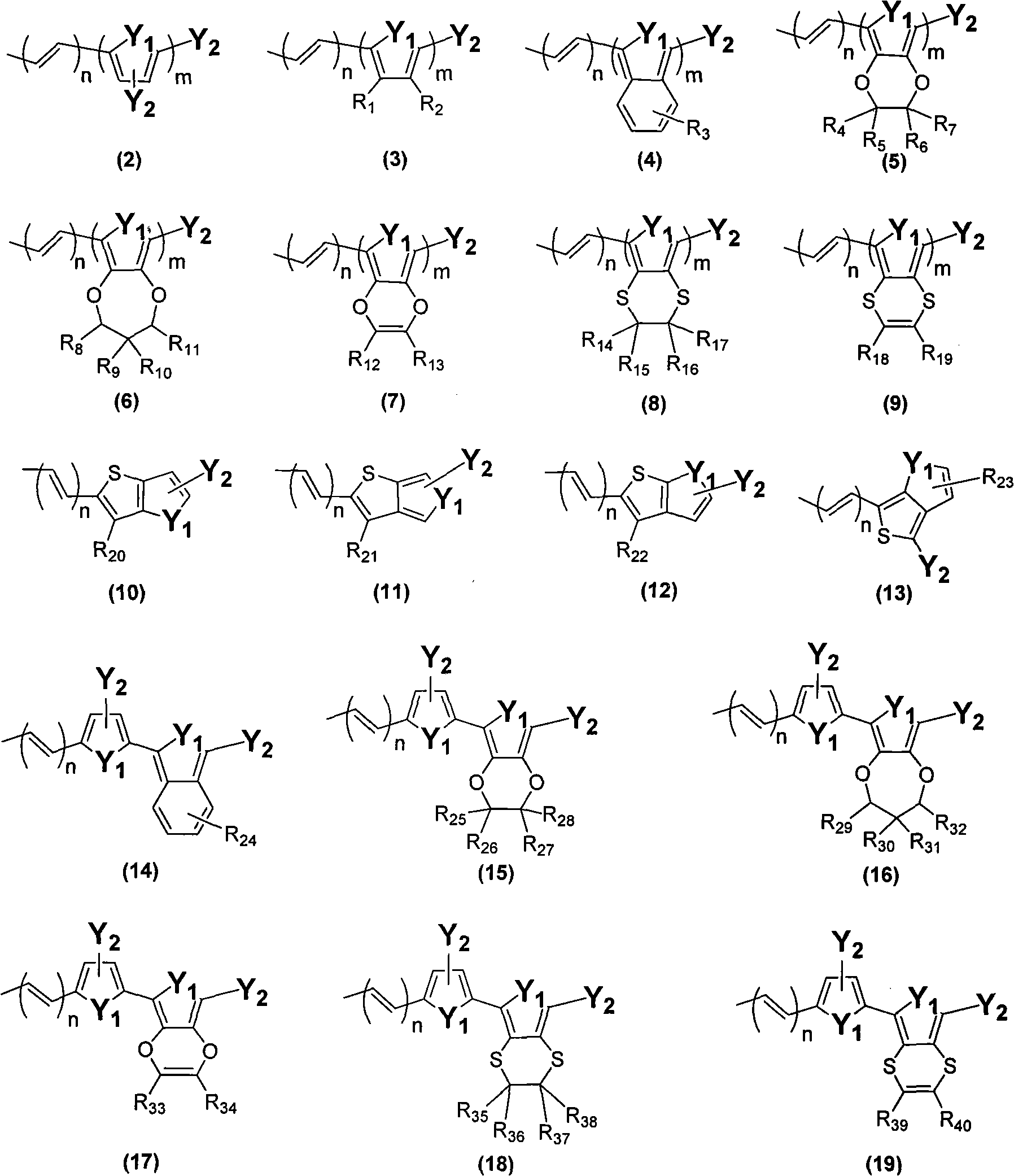
A light-sensitive coloring agent
A photosensitive dye and dye sensitization technology, which is applied in the direction of azo dyes, photosensitive equipment, organic dyes, etc., can solve the problems such as the influence of photoelectric conversion efficiency of components, and achieve the effect of excellent light absorption ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] This example is a synthetic implementation of a compound of the present invention, which is represented by CYC-B5 below.
[0080]
[0081] CYC-B5 is X in formula (1) 1 with X 2 for the same group, and X 1 Represents the group of the above formula (3), the Y of the formula (3) 1 is sulfur (S), n is 0, m is 2, R 1 with R 2 both hydrogen and Y 2 is formula (21), and C in formula (21) i h 2i+1 for C 8 h 17 . Among them, Z 1 with Z 2 for the same group, and Z 1 is a group of formula (40), wherein A 1 stands for hydrogen (H).
[0082] First, introduce the first ligand of CYC-B5 (expressed as Ligand-1, also can be expressed as 4,4'-bis(5-octyl-2.2'-bithiophen-5-yl)-2,2'-bipyridine ), the synthesis process of the first ligand is as follows.
[0083]
[0084] Among them, THF represents tetrahydrofuran (tetrahydrofuran, C 4 h 8 O), DMF represents dimethylformamide (Dimethylformamide, C 3 h 7 NO), ether is ether (C 4 h 10 O).
[0085] First, 4 g of bit...
Embodiment 2
[0100] This example is a synthetic implementation of another example of the compound of the present invention, this compound is represented by CYC-B6S below,
[0101]
[0102] CYC-B6S is X in the above formula (1) 1 with X 2 for the same group, and X 1 Represented as a group of formula (3). Among them, Y in formula (3) 1 is sulfur (S), n is 0, m is 1, R 1 with R 2 both hydrogen and Y 2 It is formula (30). R in formula (30) 46 with R 47 all C 4 h 9 ,. Among them, Z 1 with Z 2 are the same group, and Z 1 Represent the group of formula (38), and A 1 stands for hydrogen (H).
[0103] First, introduce the synthesis route of the first ligand of CYC-B6S (expressed as Ligand-6S), the synthesis process of the first ligand is as follows
[0104]
[0105] Among them, nitromethane represents nitromethane (CH 3 NO 2 ), nitrobenzene represents nitrobenzene (C 6 h 5 NO 2 ), THF means tetrahydrofuran, DMF means dimethylformamide, and ether means diethyl ether.
[...
Embodiment 3
[0118] This example is the synthesis implementation of the compound of other examples of the present invention, this compound is represented by pre-CYC-B12 below,
[0119]
[0120] pre-CYC-B12 is X in formula (1) 1 with X 2 for the same group, and X 1 Represent the group of above-mentioned formula (10), and n=0, R in formula (10) 20 is a hydrogen atom (H); Y 1 is a sulfur atom (S); and Y 2 is formula (30), and R in formula (30) 46 with R 47 all C 4 h 9 . Among them, Z 1 with Z 2 for the same group, and Z 1 is a group of formula (38), wherein A 1 stands for hydrogen (H).
[0121] First, the ligands required for the synthesis of pre-CYC-B12 (expressed as Ligand-12, can also be expressed as 4,4'-bis(3,6-di-tert-butyl-carbazol-9-yl-thieno The synthesis route of [3,2-b]thiophen-5-yl)-2,2'-bipyridine) and the synthesis process of ligand (Ligand-12) are shown below.
[0122]
[0123] First, 5.11 grams of the reaction starter (as shown in (59)) was placed in a rou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com