Acetobacter and method for producing enantiomer purity (R)-organic silanol utilizing the same
An acetobacter, enantiomer technology, applied in the field of asymmetric synthesis of biocatalytic chiral compounds, can solve the problem of low catalyst activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
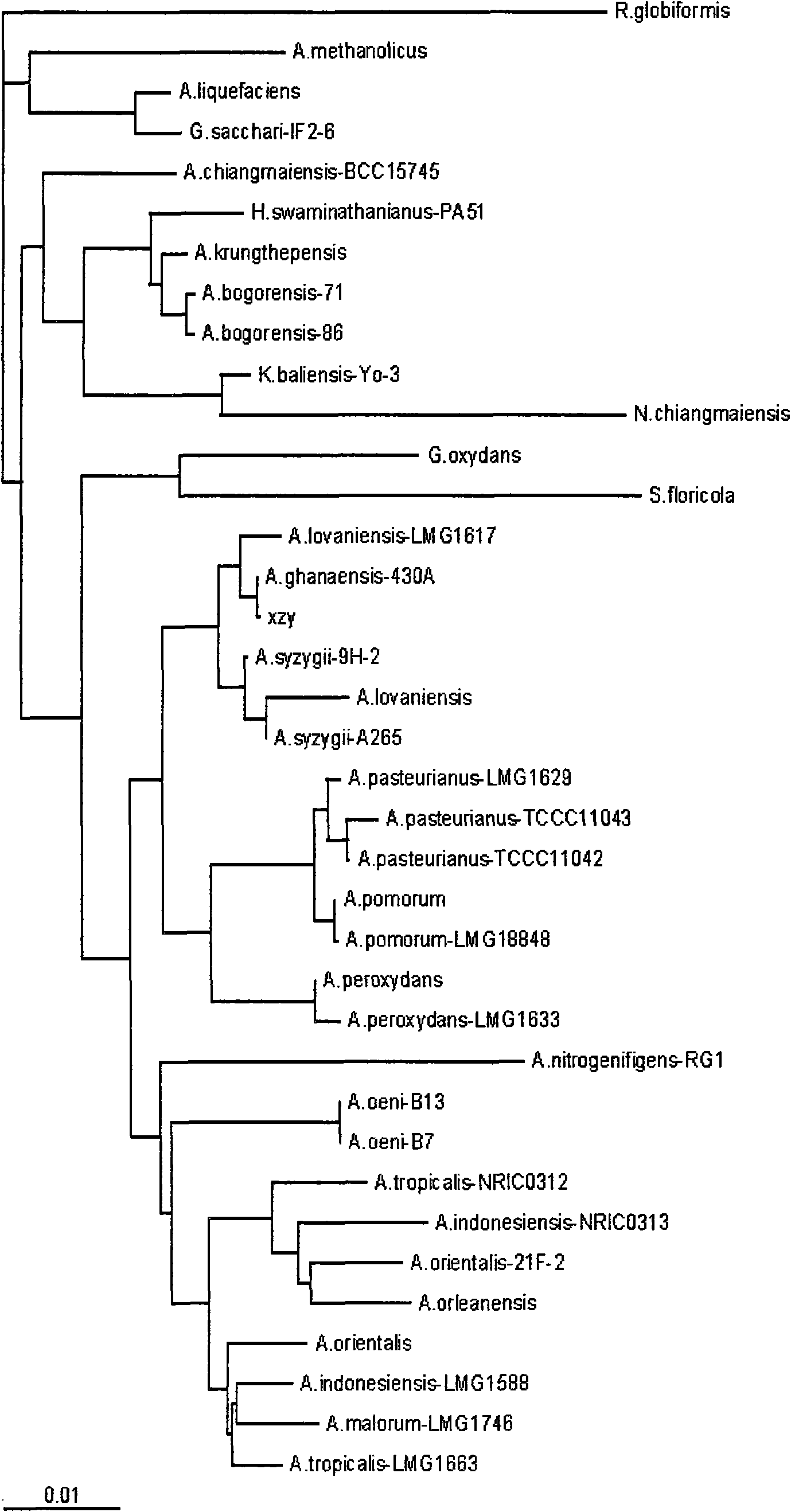
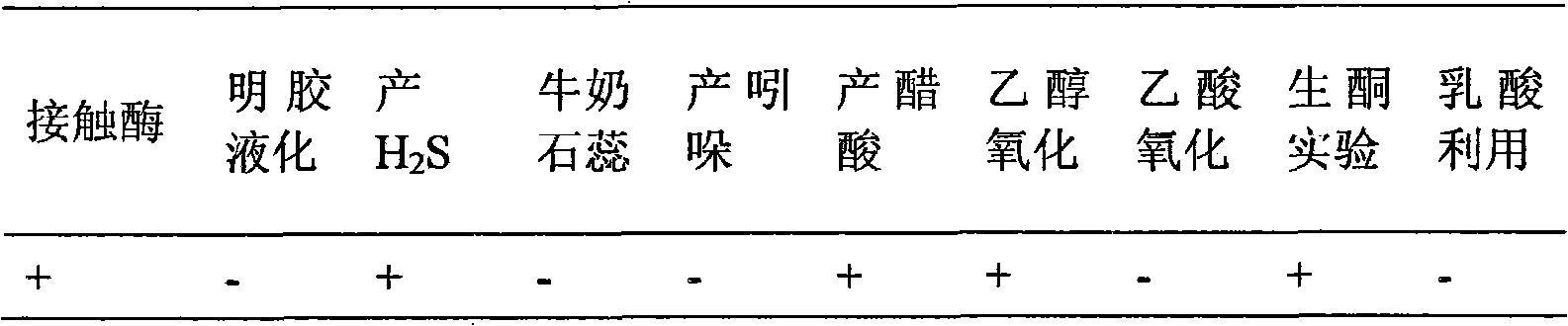
[0058] Put 2g of Chinese kefir grains into a 250mL Erlenmeyer flask filled with 100mL tomato medium, culture at 28°C for 24h, and then transfer and culture in tomato medium continuously for 3 times under the same conditions. Bacterial solution is properly diluted by the ten-fold dilution method and spread on the solid separation medium, cultivated at 28°C for 28 hours (24-36 hours can be used), and a single colony is obtained, and the slant of the purified tomato medium is preserved for identification; On March 26, 2009, it was deposited in the China Center for Type Culture Collection, CCTCCM for short, with the preservation number M209061, and the preservation address is Wuhan University, Wuhan City, Hubei Province, China (zip code 430072). The formula of the tomato medium is specifically: tomato juice 200mL / L, yeast extract 10g / L, glucose 10g / L, peptone 10g / L, pH 6.0.
[0059] The strain XZY003 identified as Acetobacter was transferred from the slant to the tomato medium, cu...
Embodiment 2
[0061]The strain XZY003 identified as Acetobacter was transferred from the slant to the tomato medium, cultivated at 30°C, 160r / min for 24h, centrifuged (8000r / min, 10min, 4°C), washed twice, and wet cells were obtained. Then disperse the wet bacteria in distilled water of equal weight, then add sodium alginate with a concentration of 10g / L 6 times the weight of wet bacteria, stir evenly, and drop the above suspension into 200mL with a syringe with a 5# needle at a concentration of 20g / L In 1 L of CaCl2 solution, harden at 4°C for 4 hours, wash the hardened calcium alginate gel balls (2-3 mm in diameter) with distilled water three times, and then transfer them into 200 mL of CaCl 2 In the glucose solution, the final concentration is 0.5g / L CaCl 2 , 200g / L glucose, placed in a refrigerator at 4°C and stored for later use, the obtained immobilized Acetobacter granules were 2-3mm in diameter, spherical, with good mechanical strength and general mechanical strength, and the cell c...
Embodiment 3
[0063] 2mL of triethanolamine hydrochloride buffer solution (triethanolamine concentration in hydrochloric acid is 0.05mol / L, pH5.0) is packed in the 10mL Erlenmeyer flask of tool stopper, then add the immobilized acetobacter granules that above-mentioned embodiment 1 prepares respectively , isopropanol and 4-trimethylsilyl-3-butyn-2-one form a mixture; the concentrations of isopropanol and 4-trimethylsilyl-3-butyn-2-one in the mixture are 43.6 mmol / L and 3.0mmol / L, the weight-to-volume ratio of the immobilized Acetobacter granules to the mixture is 0.2g / mL, react at 30°C and 180r / min for 1h to obtain (R)-4-trimethylsilane Base-3-butyn-2-ol enantiomer, gas phase detection {instrument: Japan Shimadzu GC-2010 gas chromatograph, equipped with workstation, FID detector; chiral column: HP-Chiral-5 (20% Permethylated β-cyclodextrin), column length 30m, column diameter 0.25mm (Agilent Corporation, USA). GC analysis conditions: the temperature of the vaporization chamber and the dete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com