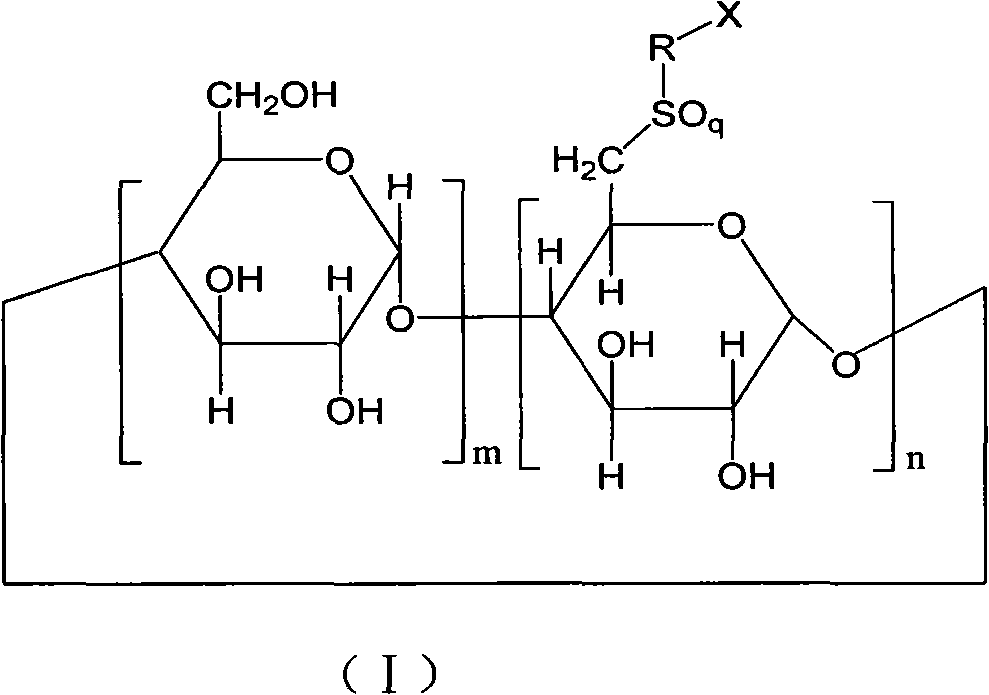
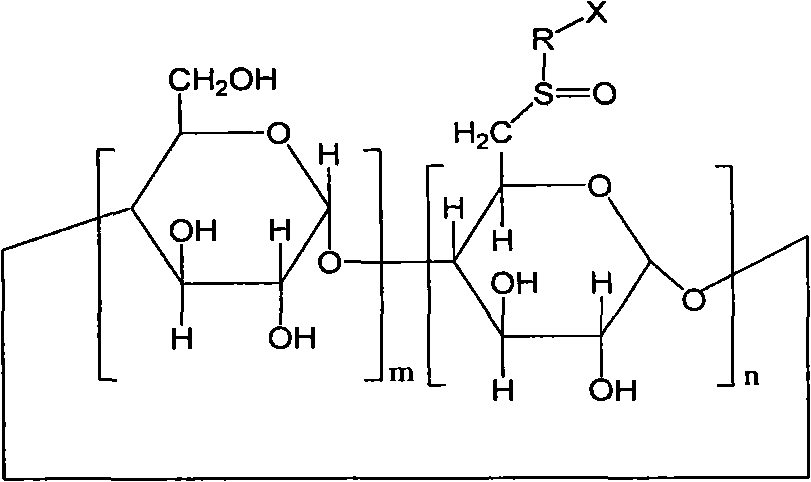
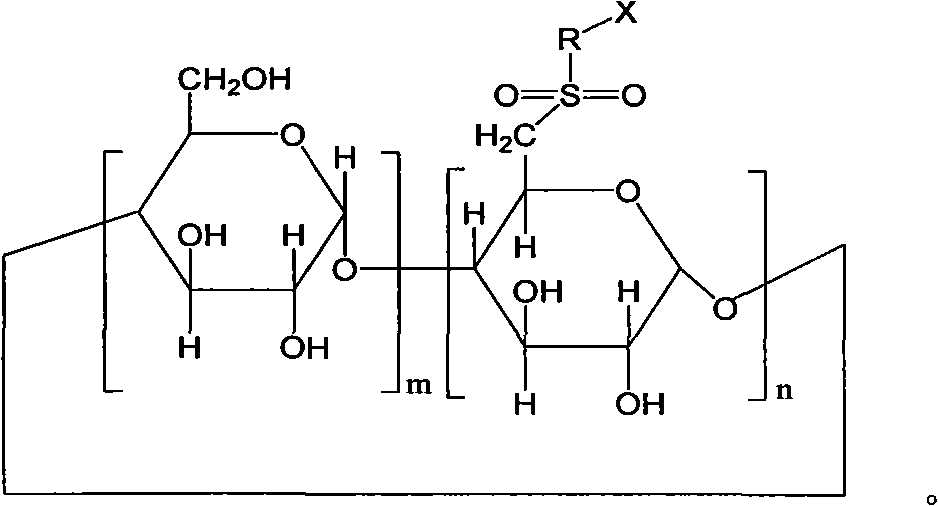
6-deoxy-sulfones cyclodextrin derivatives and preparation method thereof
A technology of deoxysulfones and cyclodextrins is applied in the field of preparing muscle relaxant antagonistic drugs, which can solve the problems of lack of specificity, limited clinical use, etc., so as to achieve the effect of reversing muscle relaxant effect, restoring neuromuscular conduction function, and ensuring life safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The synthesis of embodiment 1α-chloroacetylglycine
[0060]
[0061] Add 90g (1.2mol) of glycine to a 2000mL four-necked reaction flask, add about 400mL of water and stir to dissolve, and gradually add 64g (0.6mol) of finely ground anhydrous sodium carbonate. After adding and dissolving, cool in an ice-salt bath, add about 135.5 g (1.2 mol) of α-chloroacetyl chloride dropwise while vigorously stirring, and add sodium carbonate solution dropwise to keep the reaction solution weakly alkaline. After the addition, continue Stir for 4h, acidify to pH=1 with hydrochloric acid. Extracted twice with ethyl acetate, combined the extracts, added an appropriate amount of anhydrous sodium sulfate to dry overnight, filtered, and the filtrate was distilled under reduced pressure until crystals were precipitated, left overnight, filtered, and dried to obtain 123.6 g of colorless needle crystals, with a yield of about 68%.
Embodiment 2
[0062] Example 2 Synthesis of α-chlorophenylacetylglycine
[0063]
[0064] Add 90g (1.2mol) of glycine to a 2000mL four-necked reaction flask, add about 400mL of water and stir to dissolve, and gradually add 64g (0.6mol) of finely ground anhydrous sodium carbonate. After adding and dissolving, cool with an ice-salt bath, add about 226.85 g (1.2 mol) of α-chlorophenylacetyl chloride dropwise while vigorously stirring, and add sodium carbonate solution dropwise to keep the reaction solution weakly alkaline. Stirring was continued for 4h, acidified with hydrochloric acid to pH=1. Extracted twice with ethyl acetate, combined the extracts, added an appropriate amount of anhydrous sodium sulfate to dry overnight, filtered, and the filtrate was distilled under reduced pressure until crystals were precipitated, left overnight, filtered, and dried to obtain 180.3 colorless needle crystals, with a yield of about 66 %.
Embodiment 3
[0065] Embodiment 3 Preparation of potassium salt aqueous solution of thionicotinic acid
[0066]
[0067] (42g, 0.75mol) sodium hydrosulfide, 150ml water, stirred to dissolve. Nicotinyl chloride (49.54 g, 0.35 mol) was added dropwise under cooling in an ice bath, and the temperature was controlled not to exceed 15°C. After the dropwise addition, continue to stir for 1 hour, then cool to 0°C, add hydrochloric acid to neutralize, filter to obtain solid thionicotinic acid, then slowly add potassium carbonate water to dissolve, filter to obtain a yellow aqueous solution of thionicotinic acid potassium salt 60.6g, yield 97.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com