Alpha-asarone mixed micelle injectio and preparation method thereof
A technology of mixing micelles and Asarum, applied in the field of injections, can solve the problems of limited wide application, limited solubilization and drug loading, and insufficient stability of the system, so as to improve blood drug concentration, reduce liver distribution, and improve hydrophilicity. sex-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
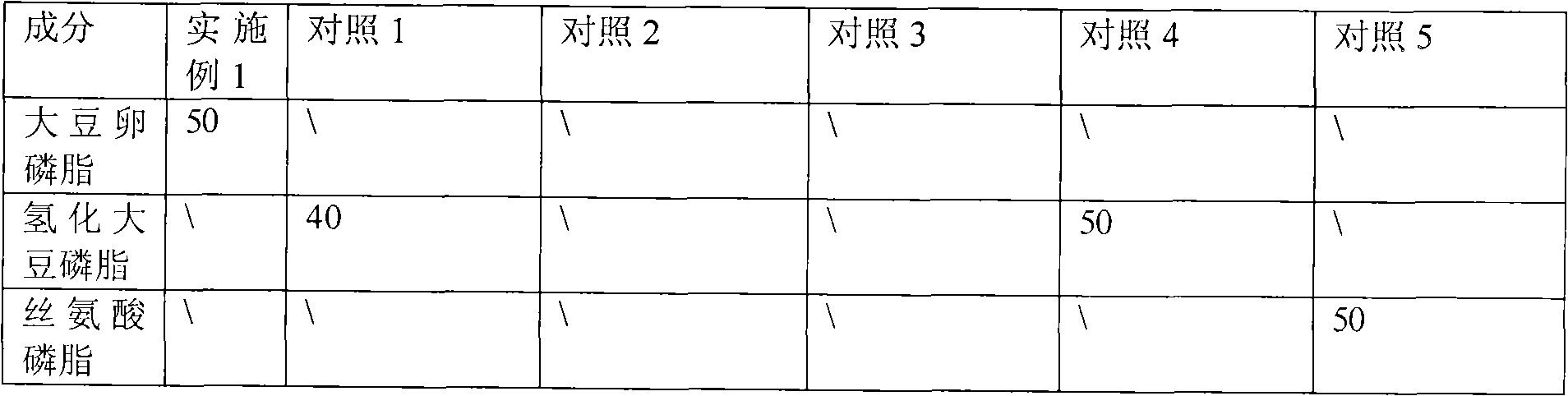
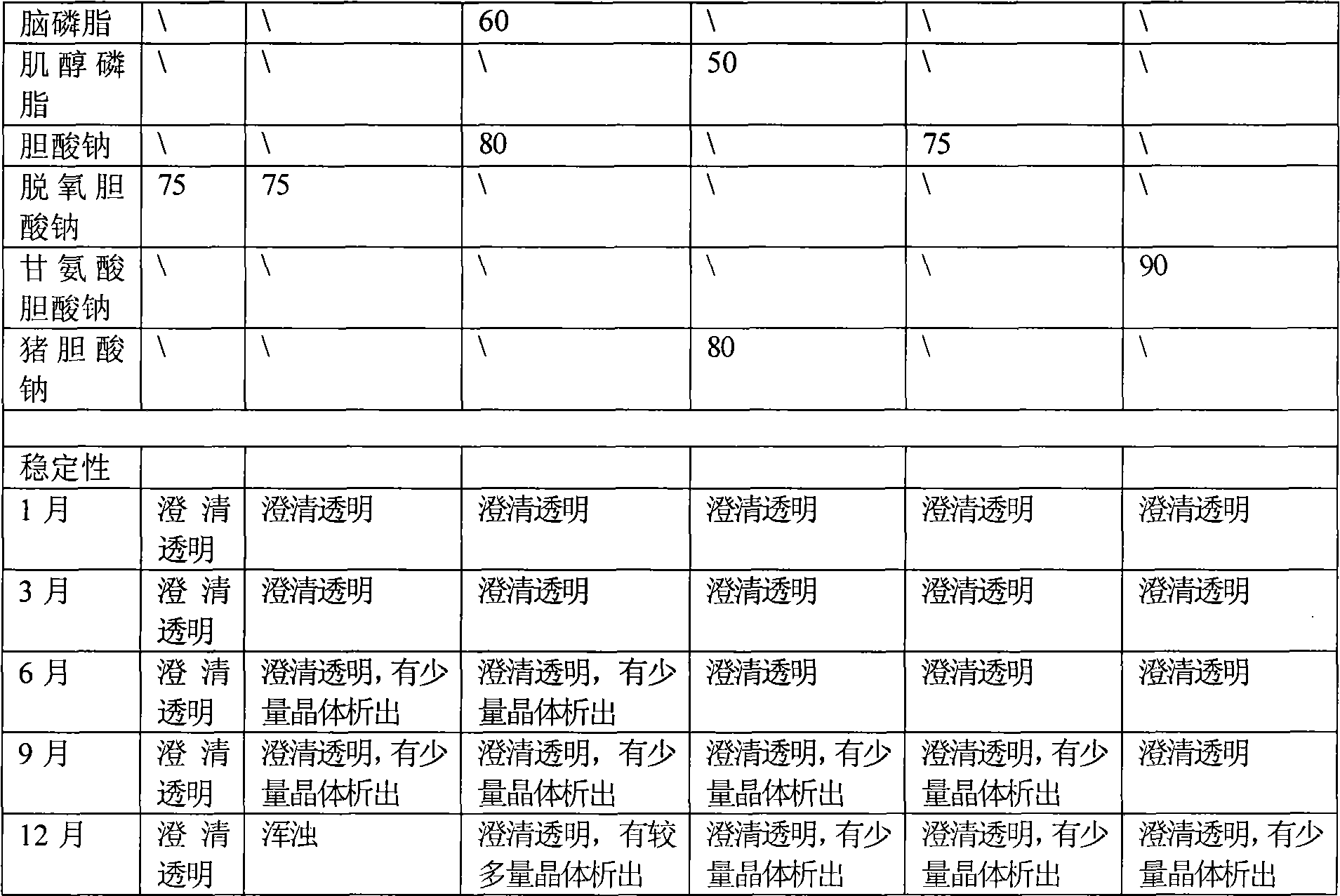
Image
Examples
Embodiment 1
[0043] Weigh 50 mg of soybean lecithin, place it in a 50 ml round bottom flask, add 7.5 ml of ethanol solution (10 mg / ml) of sodium deoxycholate, and dissolve to obtain stock solution 1. Dissolve 10 mg of α-asarone in 100 μl ethyl acetate solution to obtain stock solution 2, mix stock solution 1 and stock solution 2, and disperse evenly by ultrasonication. Then spin evaporate to remove the organic solvent, the temperature of the water bath is 35°C, spin until there is no alcohol smell, and form a transparent film, then disperse with 2.5ml of water for injection to obtain a dispersion solution containing drug-mixed micelles, centrifuge at 13000rpm / min for 5min , take the supernatant to obtain α-asarone mixed micelles injection, and then filter it with a 0.22 μm microporous membrane. The filtrate was taken to obtain α-asarone mixed micelles injection. The solubilization amount was measured to be 3.80 mg / ml, and the drug loading amount was 7.6%.
Embodiment 2
[0045] Weigh 35.7 mg of polyene phosphatidylcholine, place it in a 50 ml round bottom flask, add 9 ml of ethanol solution (10 mg / ml) of sodium glycinate cholate, and dissolve to obtain stock solution 1. Dissolve 10 mg of α-asarone in 100 μl ethanol solution to obtain stock solution 2, mix stock solution 1 and stock solution 2, and disperse evenly by ultrasonication. Rotary evaporation to remove the solvent and organic solvent, the water bath temperature is 35 ℃, spin until there is no alcohol smell, form a transparent film, and then disperse with 2.5ml of water for injection to obtain a dispersion solution containing drug mixed micelles, centrifuge at 13000rpm / min After 5 minutes, the supernatant was taken to obtain α-asarone mixed micelles injection, which was then filtered through a 0.22 μm microporous membrane. The filtrate was taken to obtain α-asarone mixed micelles injection. The measured solubilization amount is 3.65 mg / ml, and the drug loading amount is 7.3%.
Embodiment 3
[0047] Weigh 50 mg of egg yolk lecithin, place in a 50 ml round bottom flask, add 8 ml of sodium hyocholate in chloroform-methanol as a 1:1 (volume ratio) solution (10 mg / ml), dissolve, and obtain stock solution 1. Dissolve 10 mg of α-asarone in 1 ml of chloroform solution to obtain stock solution 2, mix stock solution 1 and stock solution 2, and disperse evenly by ultrasonication. The organic solvent is then removed by rotary evaporation, the water bath temperature is 45°C, and the taste of the organic solvent is spun to form a transparent film, and then 2.5ml of pH 7.4 NaH 2 PO 4 -Na 2 HPO 4 Disperse to obtain a dispersion solution of drug-containing mixed micelles, centrifuge at 13,000 rpm / min for 5 minutes, take the supernatant to obtain α-asarone mixed micelles injection, and then filter with a 0.22 μm microporous membrane. The filtrate was taken to obtain α-asarone mixed micelles injection. It was measured to be 3.67 mg / ml, with a drug loading of 7.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com