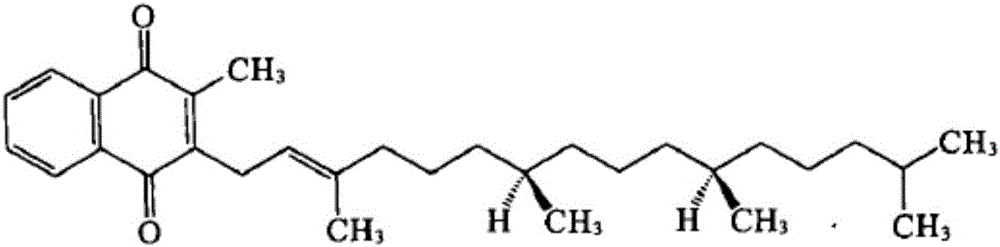
Vitamin K1 micelle injection and preparation method thereof
A vitamin and injection technology, applied in blood diseases, pharmaceutical formulations, emulsion delivery, etc., can solve the problems of insolubility, large side effects of intravenous administration, low oral bioavailability, etc., to achieve low preparation cost and reduce adverse reactions. , the effect of prolonged time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
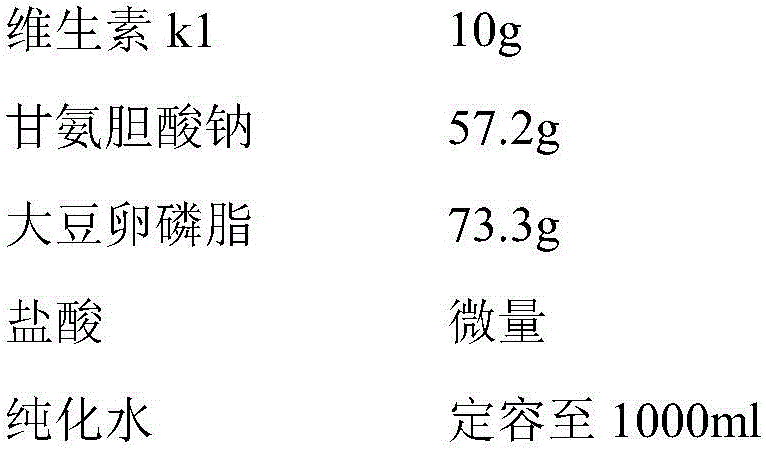
[0033] Each bottle contains vitamin K1 10mg, and the composition of 1000 bottles of vitamin K1 micellar injection is:
[0034]
[0035] The specific operation process is as follows:
[0036] (1) Weigh each component according to the ratio, add vitamin K1, sodium glycocholate, and soybean lecithin into 2-methyl-2-propanol and water (volume ratio 4:1), and stir , dissolved until clear;
[0037] (2) Put the solution in step (1) into a lyophilizer, cool down to -40°C±2°C, keep it warm for 2h to 4h, then evacuate until the pressure of the freeze-drying box is lower than 10Pa, and then use 4°C / h Raise the temperature to -25°C ± 2°C for sublimation drying, keep it warm for 10 hours, raise the temperature to 30°C ~ 35°C, keep it warm for 2 hours, freeze-drying is over, and it will be loose and dry.
[0038] (3) Add the prescribed amount of purified water to the powder, stir at room temperature until the sample is clear and transparent, adjust the pH value to 5.5-6.5 with hydrochl...
Embodiment 2
[0042] Each bottle contains vitamin K1 10mg, and the composition of 1000 bottles of vitamin K1 micellar injection is:
[0043]
[0044]
[0045] The specific operation process is as follows:
[0046] (1) Weigh each component according to the stated ratio, add vitamin K1, sodium taurocholate, and soybean lecithin to 2-methyl-2-propanol and water (volume ratio 4:1), stir, dissolve until clear;
[0047] (2) Put the solution in step (1) into a lyophilizer, cool down to -40°C±2°C, keep it warm for 2h to 4h, then evacuate until the pressure of the freeze-drying box is lower than 10Pa, and then use 4°C / h Raise the temperature to -25°C ± 2°C for sublimation drying, keep it warm for 10 hours, raise the temperature to 30°C ~ 35°C, keep it warm for 2 hours, freeze-drying is over, and it will be loose and dry.
[0048] (3) Add the prescribed amount of purified water to the powder, stir at room temperature until the sample is clear and transparent, adjust the pH value to 5.5-6.5 wi...
Embodiment 3
[0052] Each bottle contains vitamin K1 10mg, and the composition of 1000 bottles of vitamin K1 micellar injection is:
[0053]
[0054] The specific operation process is as follows:
[0055] (1) Weigh each component according to the stated ratio, add vitamin K1, sodium glycocholate, and soybean lecithin into 2-methyl-2-propanol and water (volume ratio 3:1), and stir , dissolved until clear;
[0056] (2) Put the solution in step (1) into a lyophilizer, cool down to -40°C±2°C, keep it warm for 2h to 4h, then evacuate until the pressure of the freeze-drying box is lower than 10Pa, and then use 4°C / h Raise the temperature to -25°C ± 2°C for sublimation drying, keep it warm for 10 hours, raise the temperature to 30°C ~ 35°C, keep it warm for 2 hours, freeze-drying is over, and it will be loose and dry.
[0057] (3) Add the prescribed amount of purified water to the powder, stir at room temperature until the sample is clear and transparent, adjust the pH value to 5.5-6.5 with h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com