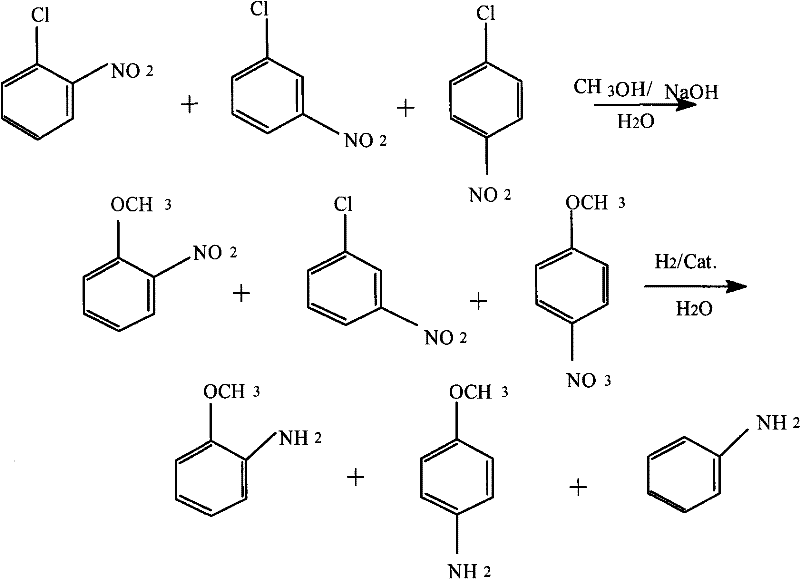
Method for producing anisidine by mixed nitrochlorobenzene reacting in aqueous solvent
A technology of aminoanisole and nitrochlorobenzene, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc., can solve the problems of increasing difficulty in environmental protection, increasing processing requirements, and increasing production costs, etc. problems, to achieve the effect of reducing equipment investment, simplifying production processes, and reducing labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] (1) etherification reaction: at 2M 3 Add mixed nitrochlorobenzene (o-nitrochlorobenzene content 36.8%, p-nitrochlorobenzene content 61.6%, m-nitrochlorobenzene 1.6%) 600kg, methyl alcohol 120kg, sodium hydroxide 200kg in the reaction kettle. Heating to 70°C, and then adding 700kg of water. Heat to 120°C, control the reaction pressure to 0.6MPa, and keep the reaction time for 4 hours. Sampling analysis showed that the total etherification conversion rate of o- and p-nitrochlorobenzene was 98.3%.
[0019] (2) Liquid-liquid separation: the reaction liquid in the previous step was cooled to room temperature (27° C.), and the water phase was separated to obtain 580 kg of etherified oil. After the water phase is filtered to remove impurities, it can be recycled for secondary batching.
[0020] (3) Hydrogenation reduction: Add 580kg of etherified oil obtained in the previous step, 1.5kg of Ce-modified Pd-C catalyst, and 50kg of water to 1M 3 In the reaction kettle, the rea...
Embodiment 2
[0025] (1) etherification reaction: at 2M 3 Add mixed nitrochlorobenzene (44.7% o-nitrochlorobenzene content, 54.1% p-nitrochlorobenzene content, 1.2% m-nitrochlorobenzene) 600kg, methanol 200kg, and sodium hydroxide 300kg in the reactor. Heat to 70°C, and then add 200kg of water. Heat to 80°C, control the reaction pressure to 0.2MPa, and keep the reaction time for 8 hours. Sampling analysis showed that the total etherification conversion rate of o- and p-nitrochlorobenzene was 97.7%.
[0026] (2) Liquid-liquid separation: the reaction liquid in the previous step was cooled to room temperature (25° C.), and the water phase was separated to obtain 586 kg of etherified oil. After the water phase is filtered to remove impurities, it can be recycled for secondary batching.
[0027] (3) Hydrogenation reduction: 583kg of etherified oil obtained in the previous step, 2.0kg of La modified Pd-C catalyst, and 250kg of water were added to 1M 3 In the reaction kettle, the reaction tem...
Embodiment 3
[0032] (1) etherification reaction: at 2M 3 Add mixed nitrochlorobenzene (65.7% o-nitrochlorobenzene content, 32.4% p-nitrochlorobenzene content, 1.9% m-nitrochlorobenzene) 600kg, methyl alcohol 120kg, sodium hydroxide 300kg in the reaction kettle. Heat to 70°C, and then add 500kg of water. Heat to 150°C, control the reaction pressure to 1.6MPa, and keep the reaction time for 4 hours. Sampling and analysis showed that the total etherification conversion rate of o- and p-nitrochlorobenzene was 99.4%.
[0033] (2) Liquid-liquid separation: the reaction liquid in the previous step was cooled to room temperature (24° C.), and the water phase was separated to obtain 586 kg of etherified oil. After the water phase is filtered to remove impurities, it can be recycled for secondary batching.
[0034] (3) Hydrogenation reduction: Add 586kg of etherified oil obtained in the previous step, 12kg of skeleton nickel catalyst, and 100kg of water to 1M 3 In the reaction kettle, the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com