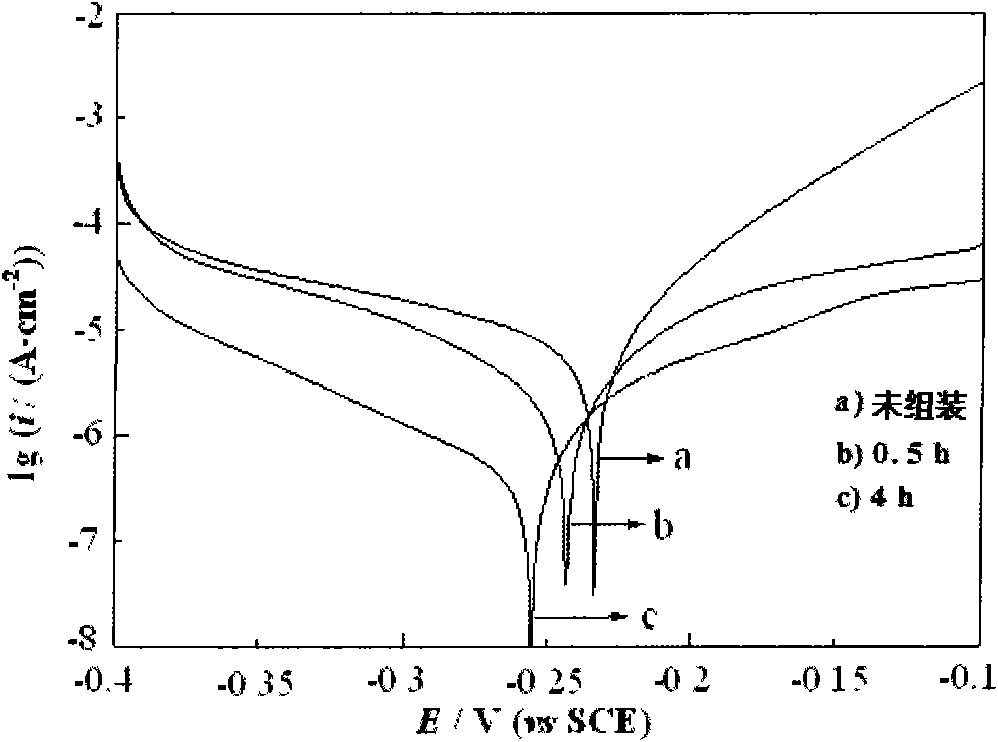
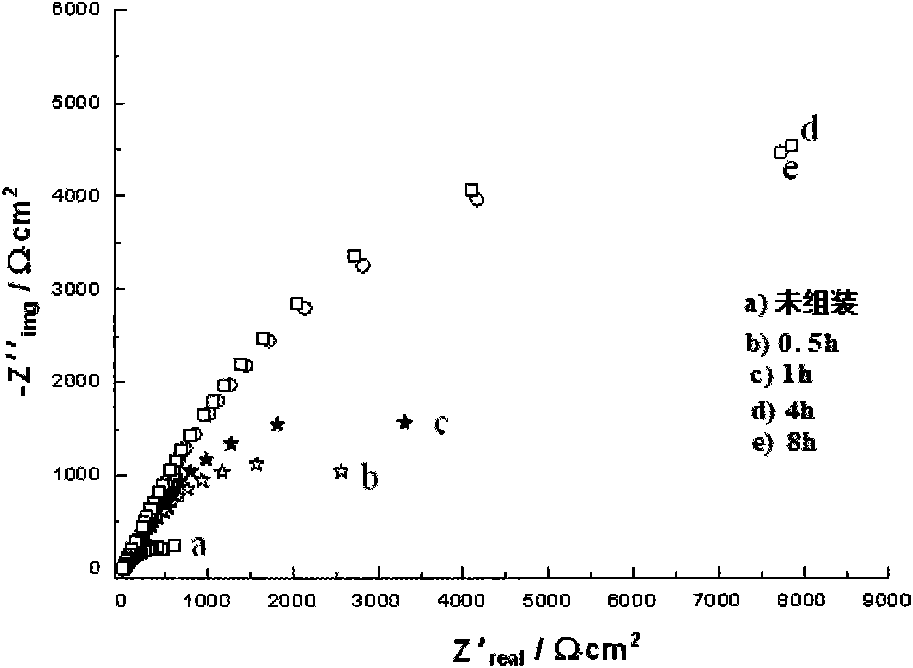
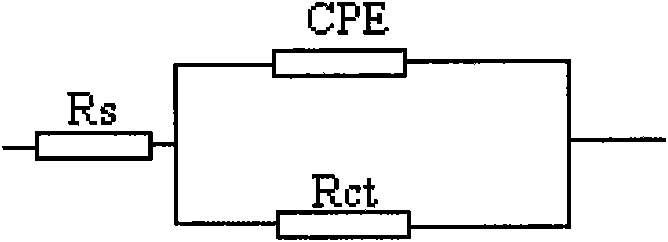
Method for forming self-assembly inhibitory film on surface of copper electrode
A copper electrode and self-assembly technology, applied in the direction of electrodes, electrolytic organic material coating, etc., can solve the problems of limited application range and low solubility, and achieve the effect of good anti-corrosion effect, simple process and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Self-assembly of anti-hydrochloric acid corrosion film on copper electrode surface
[0026] (1), the production and pretreatment of copper electrodes
[0027] Sealed with epoxy resin, the working area is 0.78cm 2 The copper electrode, the surface is 1 # ~6 # Metallographic sandpaper was polished step by step, degreased with absolute ethanol, rinsed with deionized water, and then put into the electrolytic cell. The electrolyte was 0.1mol / L KCl solution; Polarization treatment at -1.1V for 60S, repeated treatment three times for standby;
[0028] (2), configuration of self-assembly solution
[0029] Analytical pure ammonium pyrrolidinedithiocarbamate (APDTC) is formulated with a concentration of 0.01mol / L ammonium pyrrolidinedithiocarbamate (APDTC) aqueous solution;
[0030] (3) Formation of self-assembled corrosion inhibition film on copper electrode surface
[0031] Immerse the copper electrode treated in step (1) in the ammonium pyrrolidinedithiocarbamate (APDTC)...
Embodiment 2
[0033] Self-assembly of anti-hydrochloric acid corrosion film on copper electrode surface
[0034] (1), the production and pretreatment of copper electrodes
[0035] Sealed with epoxy resin, the working area is 0.78cm 2 The copper electrode, the surface is 1 # ~6 # Metallographic sandpaper was polished step by step, degreased with absolute ethanol, rinsed with deionized water, and then put into the electrolytic cell. The electrolyte was 0.1mol / L KCl solution; Polarization treatment at -1.1V for 60S, repeated treatment three times for standby;
[0036] (2), configuration of self-assembly solution
[0037] Analytical pure ammonium pyrrolidinedithiocarbamate (APDTC) is formulated with a concentration of 0.01mol / L ammonium pyrrolidinedithiocarbamate (APDTC) aqueous solution;
[0038] (3) Formation of self-assembled corrosion inhibition film on copper electrode surface
[0039] Immerse the copper electrode treated in step (1) in the ammonium pyrrolidinedithiocarbamate (APDTC)...
Embodiment 3
[0041] Self-assembly of anti-hydrochloric acid corrosion film on copper electrode surface
[0042] (1), the production and pretreatment of copper electrodes
[0043] Sealed with epoxy resin, the working area is 0.78cm 2 The copper electrode, the surface is 1 # ~6 # Metallographic sandpaper was polished step by step, degreased with absolute ethanol, rinsed with deionized water, and then put into the electrolytic cell. The electrolyte was 0.1mol / L KCl solution; Polarization treatment at -1.1V for 60S, repeated treatment three times for standby;
[0044] (2), configuration of self-assembly solution
[0045] Analytical pure ammonium pyrrolidinedithiocarbamate (APDTC) is formulated with a concentration of 0.01mol / L ammonium pyrrolidinedithiocarbamate (APDTC) aqueous solution;
[0046] (3) Formation of self-assembled corrosion inhibition film on copper electrode surface
[0047] Immerse the redox pretreated copper electrode into the 0.01mol / L ammonium pyrrolidinedithiocarbamat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com