Use of 1,2,3,4,6-penta-O-galloyl-b-D-glucose in preparation of anti-flu drugs
A technology of galloyl group and glucose, applied in the field of food or cosmetic preparation of anti-influenza virus drugs, can solve problems such as drug resistance and influenza virus mutation, and achieve the effect of strong antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
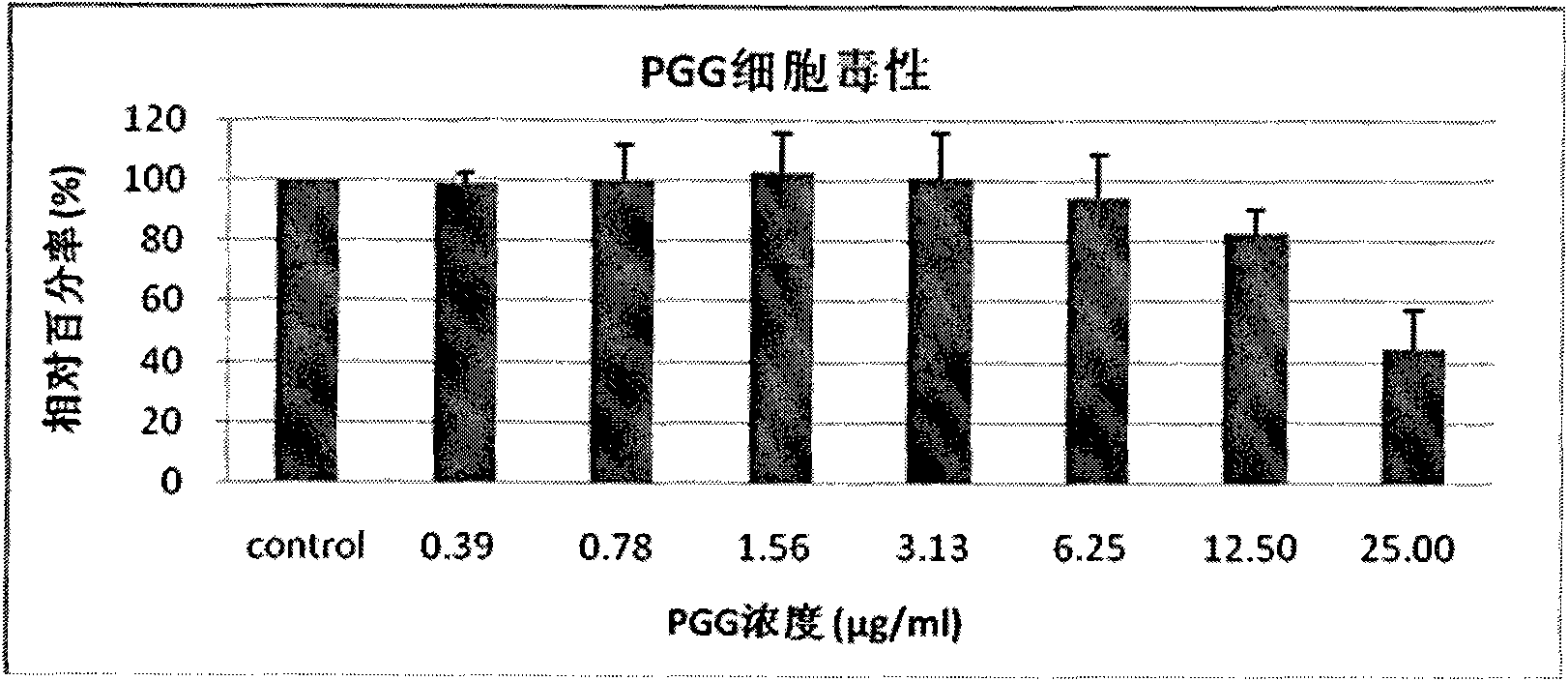
[0037] Example 1: Detection of cytotoxicity and anti-influenza virus activity of PGG
[0038] (1) The cytotoxicity detection method of PGG is as follows: 3×10 4 MDCK cells / well were inoculated in a 96-well plate, and cultured in MEM medium containing 10% (v / v) fetal bovine serum (containing penicillin 100 U / ml and streptomycin 100 μg / ml) for 24 hours. , remove the medium by suction, add 200 μl of MEM medium (penicillin 100U) containing vitamins with a volume percentage concentration of 1% (v / v) containing serially diluted concentrations (0.39 μg / ml-25 μg / ml, 2-fold dilution) PGG / ml, streptomycin 100μg / ml), at 37°C, 5% CO 2 After culturing in the incubator for 72 hours, the WST-1 method was used for detection. Three replicate wells were set up for each experimental group, and the experiment was repeated four times.
[0039] WST-1 method: Add 10 μl of WST-1 solution to each well of the 96-well plate after 72 hours of culture, at 37 ° C, 5% CO 2 After culturing in the incuba...
Embodiment 2
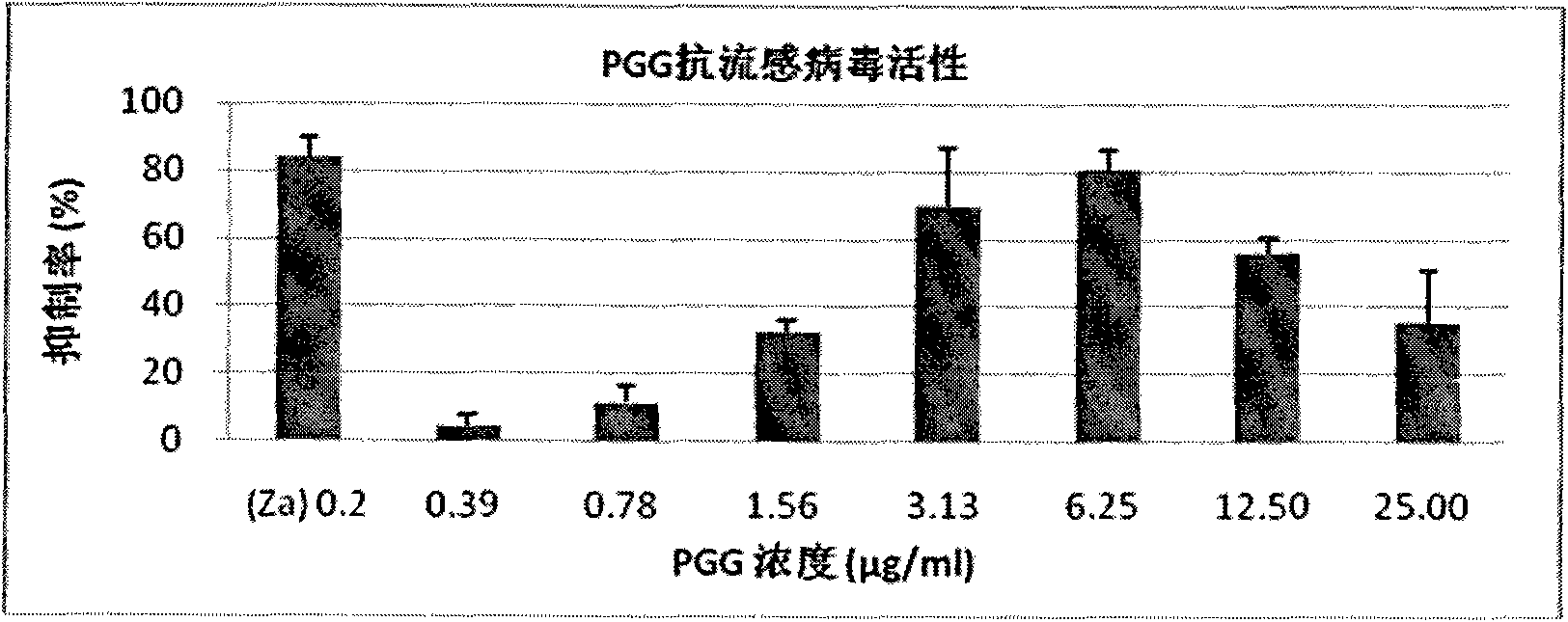
[0044] Example 2: Inhibitory Effect of Different PGG Concentrations on Influenza Virus Replication in MDCK Cells
[0045] Experimental method: put 8×10 5 MDCK cells / well were inoculated in a 12-well plate, and cultured in MEM medium (penicillin 100 U / ml, streptomycin 100 μg / ml) containing 10% (v / v) fetal bovine serum by volume for 24 hours. After washing the cells twice with phosphate buffer (PBS(-)), add influenza virus A / WSN / 33 (H1N1 ) 200 μl (virus infection dose is MOI=0.001). at 37°C, 5% CO 2 After infection in the incubator for 1 hour, the virus infection solution was sucked and discarded, the cells were washed twice with phosphate buffer, and PGG containing different concentrations (1.56 μg / ml-12.5 μg / ml, 2-fold dilution) was added with a volume percentage concentration of 1% (v / v) vitamin MEM medium (containing 100 U / ml of penicillin and 100 μg / ml of streptomycin), continued culturing for 48 hours and recovered the culture supernatant. The virus shed in the culture...
Embodiment 3
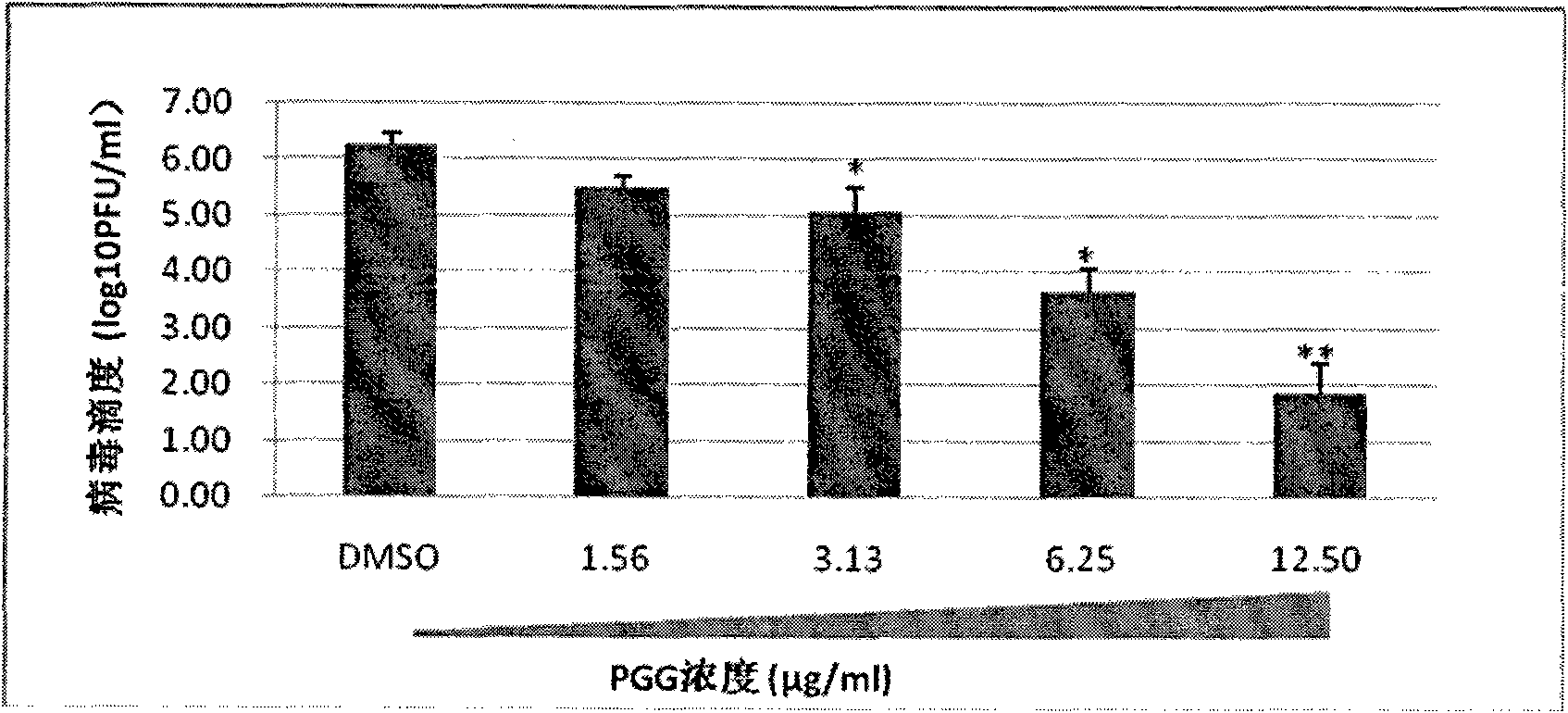
[0049] Example 3: PGG inhibits the replication of influenza virus in MDCK cells
[0050] Experimental method: put 8×10 5 MDCK cells / well were inoculated in a 12-well plate, and cultured for 24 hours in MEM medium (containing penicillin 100 U / ml and streptomycin 100 μg / ml) containing fetal bovine serum (10% (v / v) by volume concentration); After washing the cells twice with PBS (-), add 200 μl of influenza virus A / WSN / 33 (H1N1) diluted with serum-free MEM medium (containing penicillin 100 U / ml, streptomycin 100 μg / ml) to each well (virus infection Doses are at MOI=0.001). at 37°C, 5% CO 2 After infection in the incubator for 1 hour, draw and discard the virus infection solution, wash the cells twice with PBS(-), add 2ml of PGG containing different concentrations (1.56μg / ml~12.5μg / ml, 2 times dilution) containing volume percentage concentration The MEM culture medium (containing penicillin 100U / ml, streptomycin 100 μg / ml) that is 1% vitamin; Experimental group (add containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com