Method for inhibiting protein intron activity and application thereof in medicine preparation
A protein intron, active technology, applied in the direction of organic active ingredients, antibacterial drugs, pharmaceutical formulations, etc., to achieve the effect of simplifying clinical trial work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
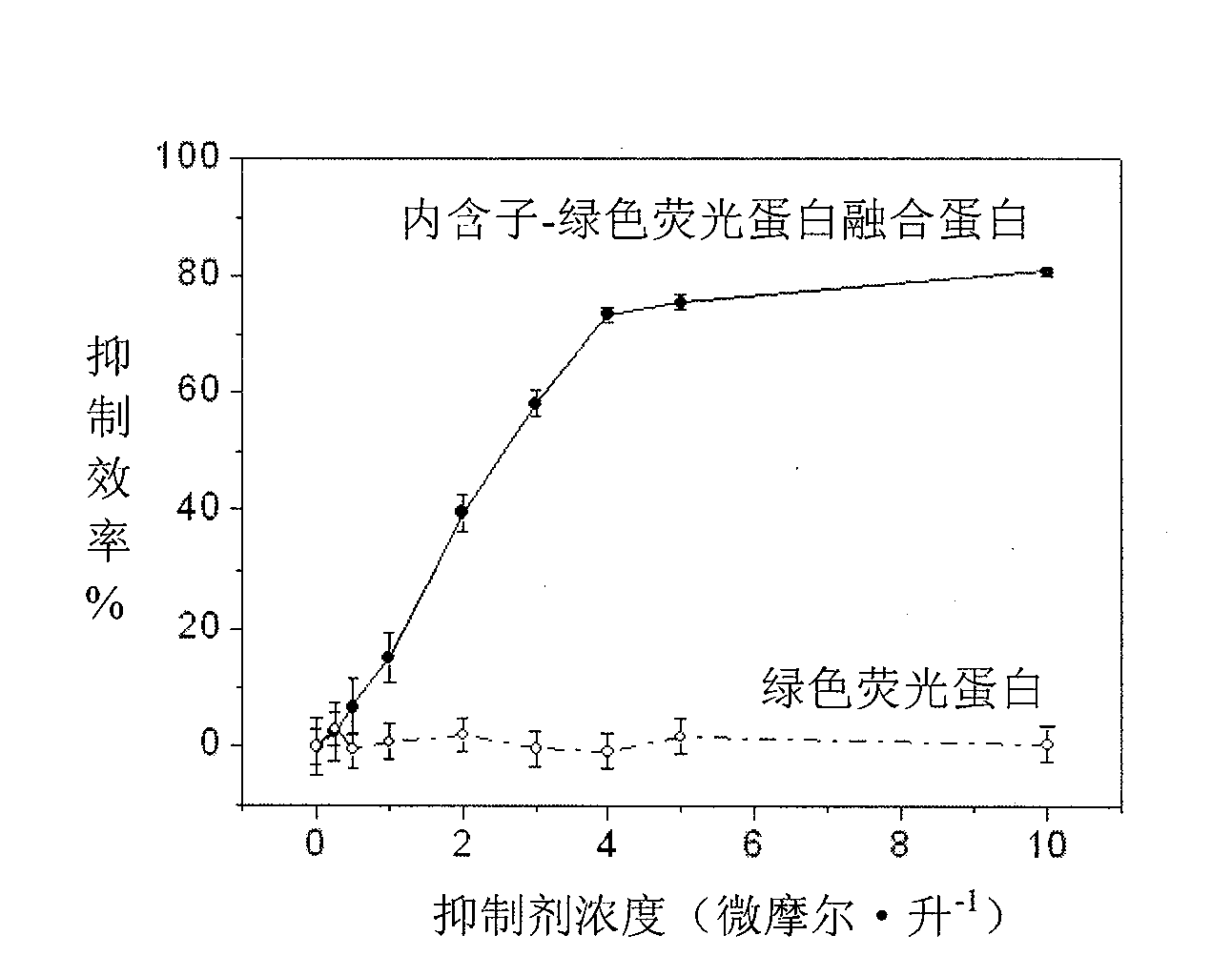
[0037] The fusion protein of RecA Intein and green fluorescent protein GFP in Mycobacterium tuberculosis was expressed by Escherichia coli. Due to the insertion of RecA Intein, the luminescent effect of the green fluorescent protein GFP is completely eliminated. If RecA Intein can exert its protein splicing function and connect the two ends of the GFP protein, the luminescence of the fluorescent protein is restored. The presence of intein activity inhibitors will suppress fluorescence. Thus, the change in fluorescence intensity can be used to detect the efficiency of the intein activity inhibitor.
[0038] We dissolve the fusion protein from the inclusion body with complex buffer (8M urea, 0.5M sodium chloride, 20mM sodium phosphate, pH=7.5), then adsorb the fusion protein through a metal affinity column and elute other foreign proteins to remove it. The phosphate buffer containing 0.5M sodium chloride was used for step-by-step gradient elution. After the urea was completely...
Embodiment 2
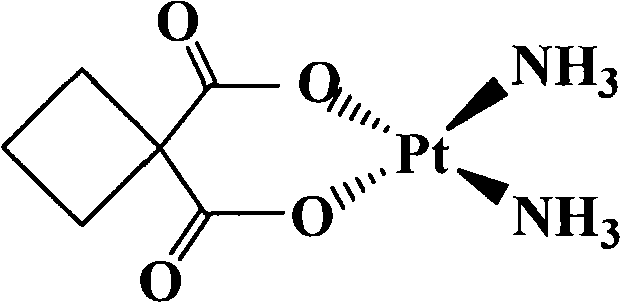
[0044] The specific experimental method and principle are the same as in Example 1, and the inhibitory effect of the platinum complex diaminocyclobutane dihydroxy acid platinum is detected, and its IC 50 About 30μM. The structural formula of platinum dihydrocyclobutane dihydroxy acid is:
[0045]
Embodiment 3
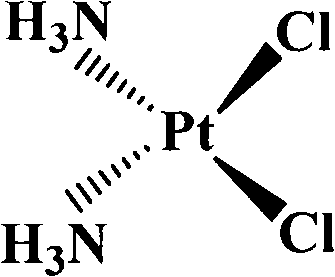
[0047] Concrete experimental method and principle are with embodiment 1, detect the platinum compound chloride monochlorine diamine imidazolium platinum ([cis-Pt(NH 3 ) 2 (im)Cl]Cl) inhibition, its IC 50 About 77μM. The structural formula of chlorine chloride diamine imidazolium platinum is:
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com