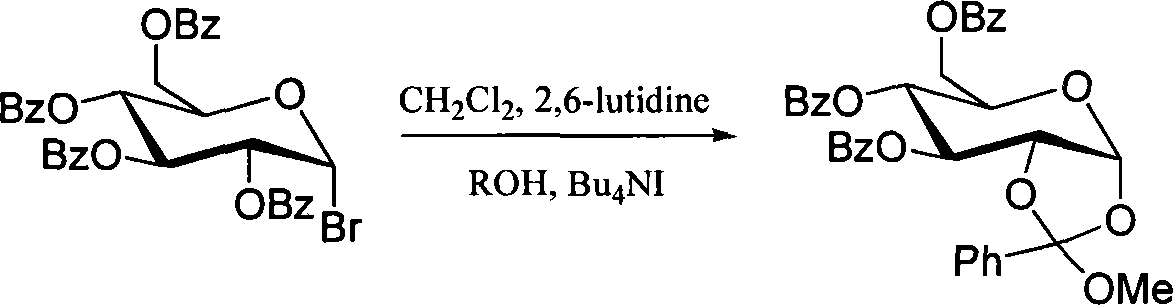
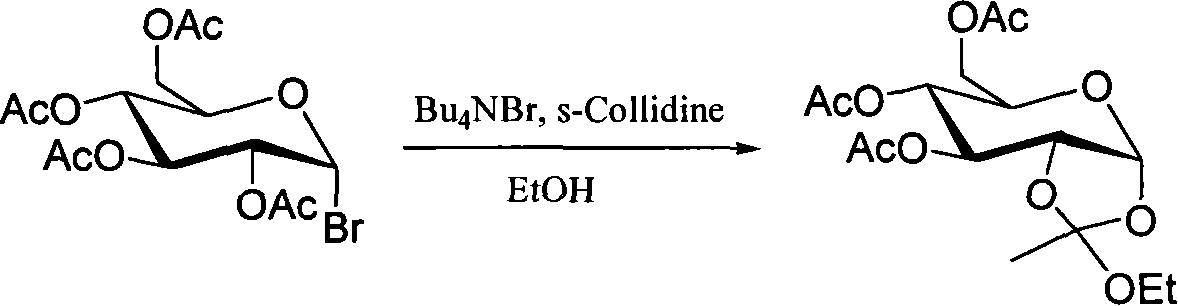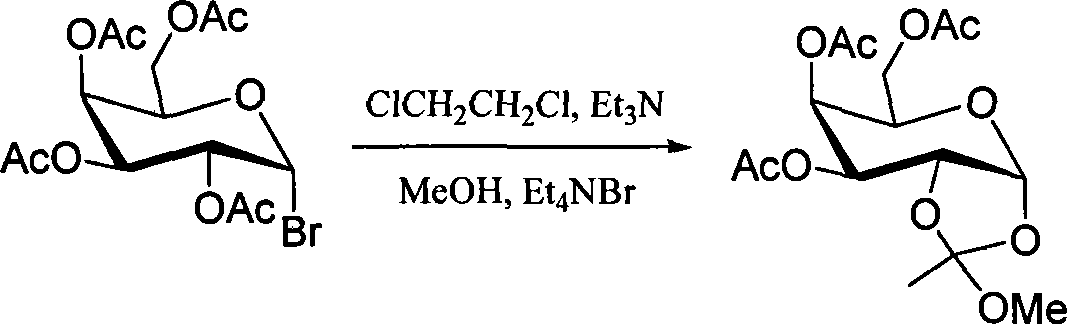Method for preparing sugar ortho ester
A technology of sugar orthoester and monosaccharide, applied in the field of preparation of sugar orthoester, can solve the problems of large environmental pollution and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Add 57mg 2,3,4,6-tetra-O-acetylbromoglucopyranose and 0.57mL acetonitrile to a 10mL round bottom flask, then add 45mg tetrabutylammonium bromide, 46mg sodium bicarbonate , 0.056mL methanol and 57mg Molecular sieves, stirred at 40°C for 3 hours. After the reaction was detected by TLC, it was directly concentrated under reduced pressure and purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate 3:2) to obtain pure 3,4,6-tri-O-acetylpyran Glucose-1,2-(methyl orthoester) 43 mg, yield 86%.
Embodiment 2
[0026] Example 2: Add 57mg 2,3,4,6-tetra-O-acetylbromoglucopyranose and 0.57mL acetonitrile to a 10mL round bottom flask, then add 45mg tetrabutylammonium bromide, 23mg sodium bicarbonate , 0.056mL methanol and 57mg Molecular sieves, stirred at 40°C for 6 hours. After the reaction was detected by TLC, it was directly concentrated under reduced pressure and purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate 3:2) to obtain pure 3,4,6-tri-O-acetylpyran Glucose-1,2-(methyl orthoester) 42 mg, yield 84%.
Embodiment 3
[0027] Example 3: Add 57mg 2,3,4,6-tetra-O-acetylbromoglucopyranose and 0.57mL acetonitrile to a 10mL round bottom flask, then add 13mg tetrabutylammonium bromide, 23mg sodium bicarbonate , 0.056mL methanol and 57mg Molecular sieves, stirred at 40°C for 6 hours. After the reaction was detected by TLC, it was directly concentrated under reduced pressure and purified by silica gel column chromatography (eluent: petroleum ether / ethyl acetate 3:2) to obtain pure 3,4,6-tri-O-acetylpyran Glucose-1,2-(methyl orthoester) 40 mg, yield 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com