Felodipine sustained-release preparation and preparation method thereof
A felodipine, slow-release preparation technology, applied in the field of medicine, can solve the problem of no negative inotropic effect found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
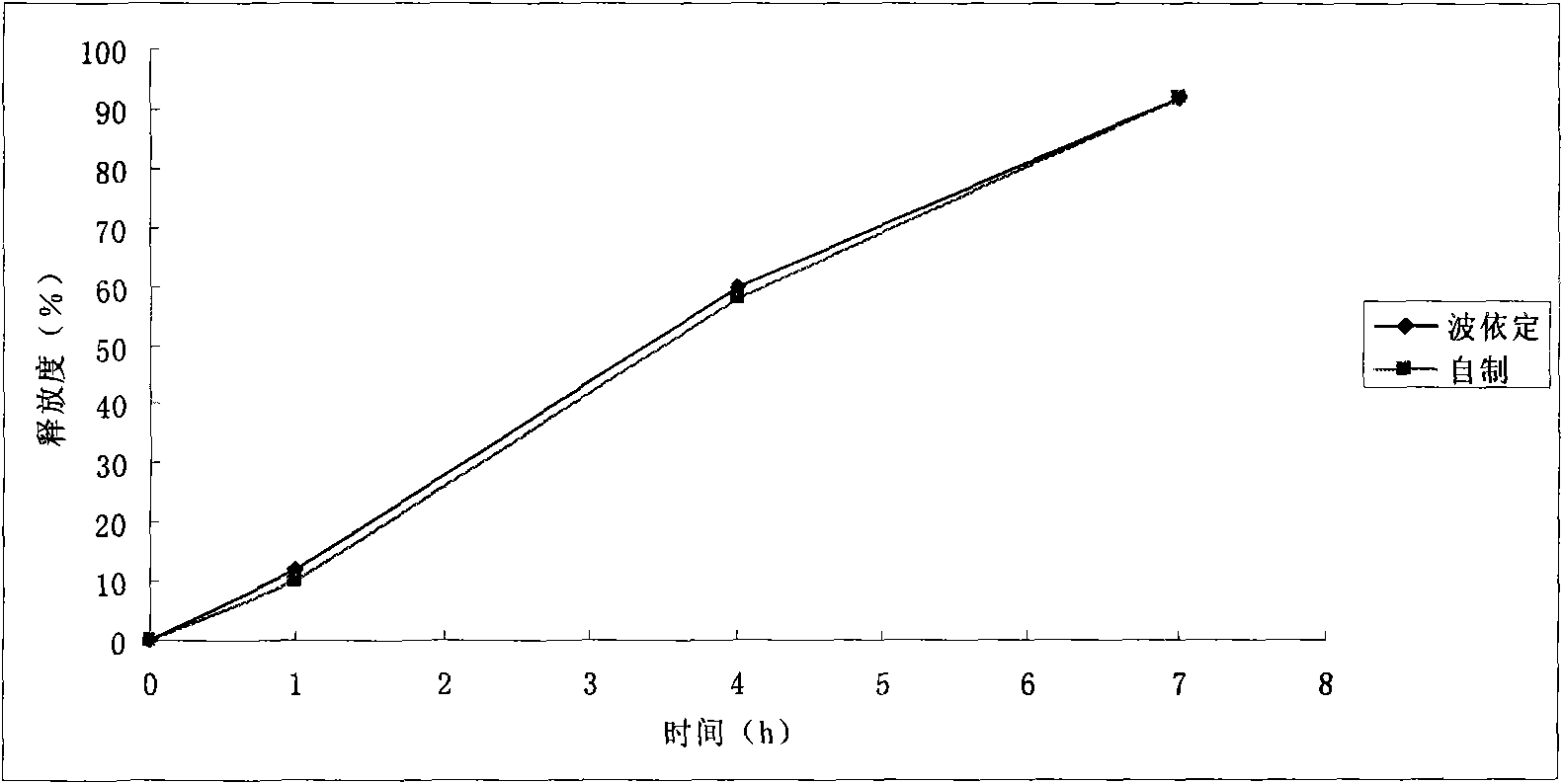
Embodiment 1
[0067] prescription:
[0068] Plain Tablet Prescription:
[0069] Felodipine 5g
[0070] Copovidone 20g
[0071] Microcrystalline Cellulose 41
[0072] Sucrose 82g
[0073] Hypromellose 50g
[0075] Proper amount of ethanol
[0076] Coating Solution Prescription:
[0077] Opadry 30g
[0078] Appropriate amount of water
[0079] Makes 1000 pieces
[0080] Preparation process:
[0081] 1 Preparation process of plain tablets
[0082] 1.1 Preparation of the main agent-containing adhesive
[0083] Take 5 g of felodipine and add 60 g of ethanol and stir to dissolve, then add 20 g of copovidone and stir to dissolve, and place it away from light for later use.
[0084] 1.2 Granulation
[0085] Crush the sucrose, pass it through a 80-mesh sieve, and put it into a wet mixing granulator together with microcrystalline cellulose and hypromellose, stir to mix evenly, and then add the binder containing the main ingredient to granulate , Rinse the...
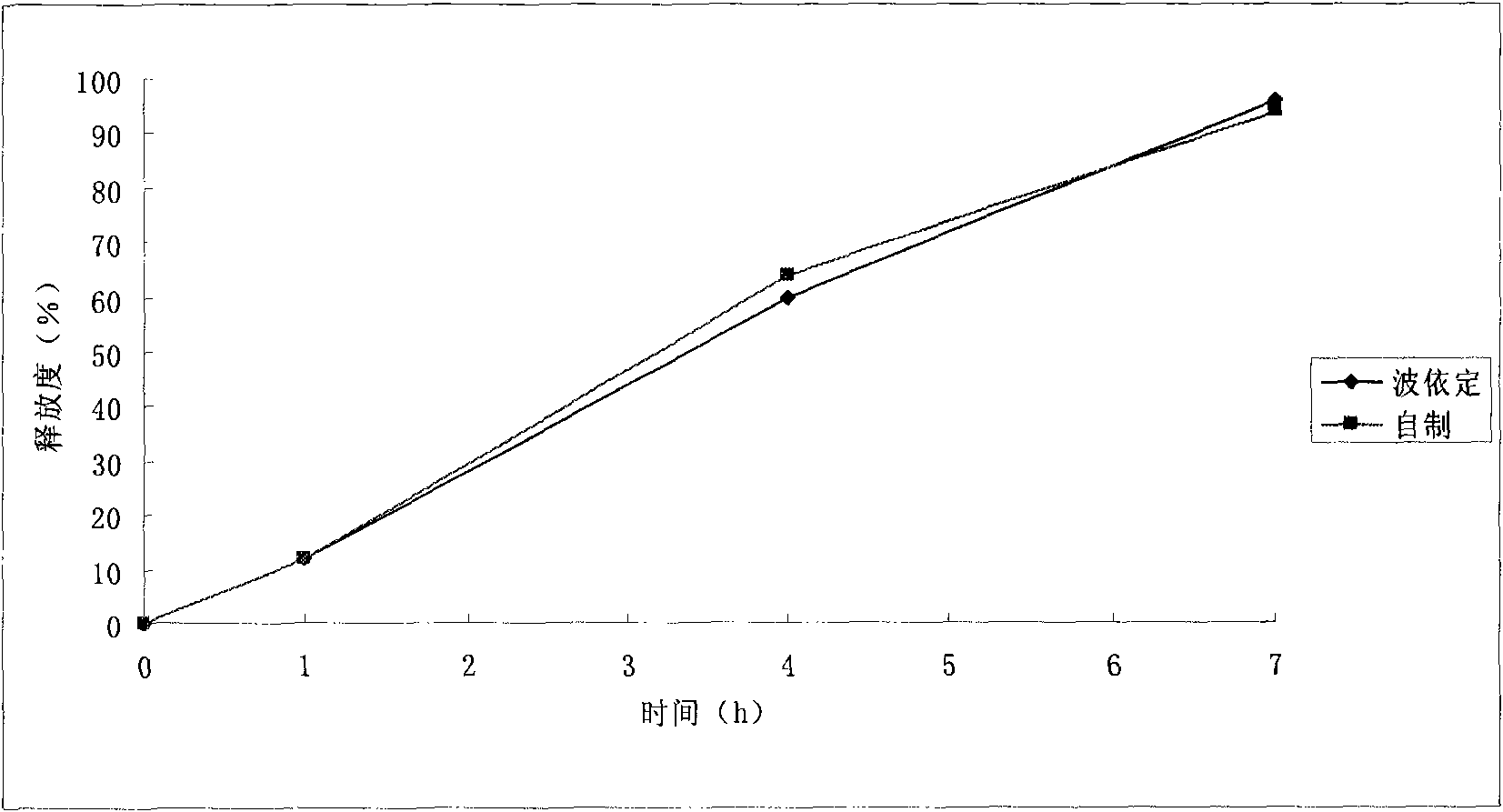
Embodiment 2
[0094] prescription:
[0095] Plain Tablet Prescription:
[0096] Felodipine 5g
[0097] Povidone 20g
[0098] Microcrystalline Cellulose 40g
[0099] Sucrose 40g
[0100] Hypromellose 40g
[0101] Starch 40g
[0103] Proper amount of ethanol
[0104] Coating Solution Prescription:
[0105] Opadry 20g
[0106] Appropriate amount of water
[0107] Makes 1000 pieces
[0108] Preparation process:
[0109] 1 Preparation process of plain tablets
[0110] 1.1 Preparation of the main agent-containing adhesive
[0111] Take 5g of felodipine and add 70g of ethanol and stir to dissolve, then add 20g of povidone and stir to dissolve, and place it away from light for later use.
[0112] 1.2 Granulation
[0113] Crush the sucrose, pass it through a 80-mesh sieve, and put it into a wet mixing granulator together with microcrystalline cellulose, hypromellose, and starch, stir to mix evenly, and add the binder containing the main ingredient after mi...
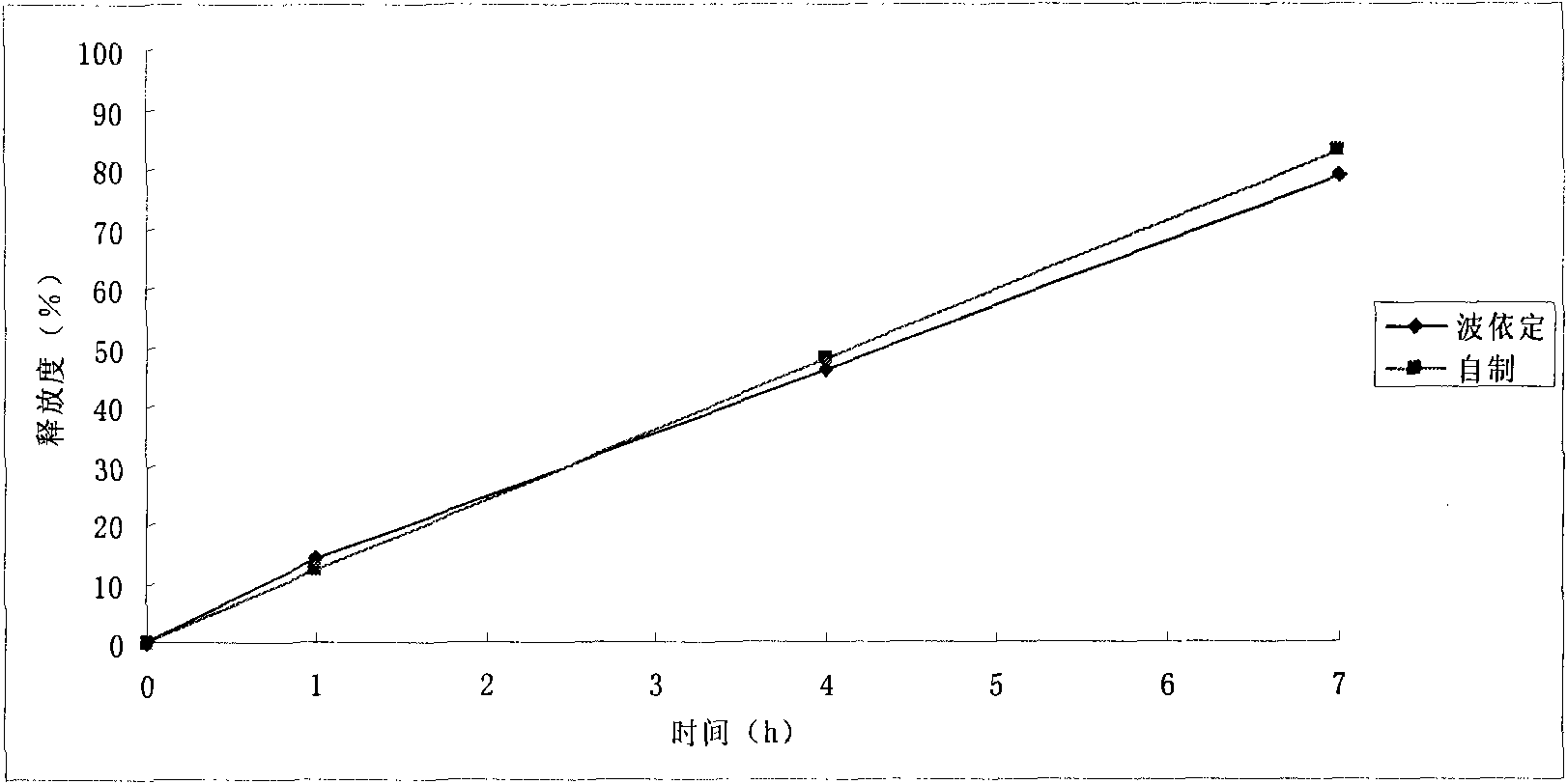
Embodiment 3
[0122] prescription:
[0123] Plain Tablet Prescription:
[0124] Felodipine 5g
[0125] Copovidone 20g
[0126] Starch 40g
[0127] Sucrose 52g
[0128] Hypromellose 75g
[0129] Micronized silica gel 5g
[0130] Stearic acid 2g
[0131] Proper amount of ethanol
[0132] Coating Solution Prescription:
[0133] Opadry 20g
[0134] Appropriate amount of water
[0135] Makes 1000 pieces
[0136] Preparation process:
[0137] 1 Preparation process of plain tablets
[0138] 1.1 Preparation of the main agent-containing adhesive
[0139] Take 5 g of felodipine and add 53 g of ethanol and stir to dissolve, then add 20 g of copovidone and stir to dissolve, and place it away from light for later use.
[0140] 1.2 Granulation
[0141] The sucrose is crushed and passed through an 80-mesh sieve for later use. Take the prescribed amount of sucrose powder and micropowder silica gel to pre-mix through a 40-mesh sieve, and then put them into a wet mixing granulator together wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com