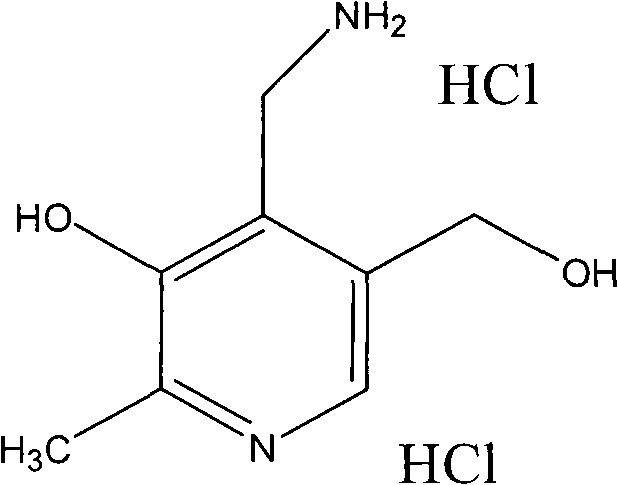
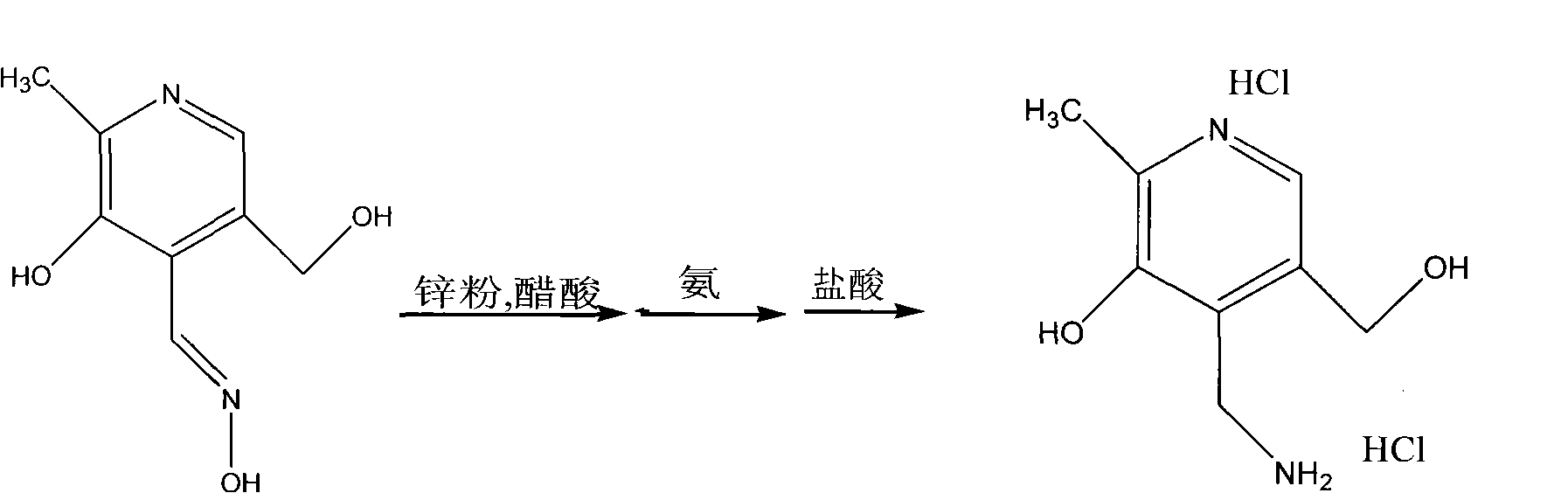
Preparation method of pyridoxamine dihydrochloride
A technology of pyridoxamine dihydrochloride and pyridoxamine, which is applied in the field of preparation of pyridoxamine dihydrochloride, can solve the problems of large solvent consumption and high cost, reduce preparation cost, save organic raw materials and solvents Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A) preparation of pyridoxine:
[0027] First add 100g (0.486mol) pyridoxine hydrochloride and 750g pure water in the 2000ml four-necked reaction flask equipped with stirring device and thermometer, stir at room temperature, pyridoxine hydrochloride is dissolved, then add active 75g (0.863mol) of manganese dioxide and 90g (0.918mol) of concentrated sulfuric acid were added dropwise, the reaction temperature was controlled at 40-45°C, and the time for adding the concentrated sulfuric acid was 45min. 48 DEG C of reaction, by TLC monitoring reaction, after the pyridoxine hydrochloride reaction as starting material is complete, obtain active substance pyridoxal, then add anhydrous sodium acetate 150g (1.83mol) and hydroxylamine hydrochloride 59.4g (0.855 mol) reaction, the reaction temperature is 55-60 ° C, reacted under stirring for 30 minutes, and finally through cold analysis, filtration and drying, 81.4 g of pyridoxine was obtained, the molar yield was 92%, and the chemi...
Embodiment 2
[0035] A) preparation of pyridoxine:
[0036] First add 50g (0.243mol) pyridoxine hydrochloride and 400g pure water in the 1000ml four-necked reaction flask equipped with stirring device and thermometer, stir at room temperature, pyridoxine hydrochloride is dissolved, then add active 37.5g (0.43mol) of manganese dioxide and 45g (0.46mol) of concentrated sulfuric acid were added dropwise, the reaction temperature was controlled at 40-45°C, and the time for adding concentrated sulfuric acid was 45min. -48 DEG C of reaction, by TLC monitoring reaction, treat after the pyridoxine hydrochloride reaction as starting material is complete, obtain active substance pyridoxal, then add anhydrous sodium acetate 75g (0.915mol) and hydroxylamine hydrochloride 29.7g ( .428mol) reaction, reaction temperature is 55-60 ℃, reacts 30min under stirring state, finally through cold analysis, filtration and drying, obtains pyridoxine 40.8g, and molar yield is 92%, and chemical purity is 99.5%. This ...
Embodiment 3
[0040] A) preparation of pyridoxine:
[0041] First add 120g (0.583mol) pyridoxine hydrochloride and 900g pure water in the 2000ml four-necked reaction flask that stirring device and thermometer are housed, stir at room temperature, pyridoxine hydrochloride is dissolved, then add active 90g (1.03mol) of manganese dioxide and 108g (1.1mol) of concentrated sulfuric acid were added dropwise, the reaction temperature was controlled at 40-45°C, and the time for adding the concentrated sulfuric acid was 45min. 48 DEG C of reaction, by TLC monitoring reaction, after the pyridoxine hydrochloride reaction as starting material is complete, obtain active substance pyridoxal, then add anhydrous sodium acetate 180g (2.2mol) and hydroxylamine hydrochloride 71.3g (1.03 mol) reaction, the reaction temperature is 55-60 ° C, and the reaction is carried out under stirring for 30 minutes, and finally through cold analysis, filtration and drying, 98.0 g of pyridoxine is obtained, the molar yield i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com