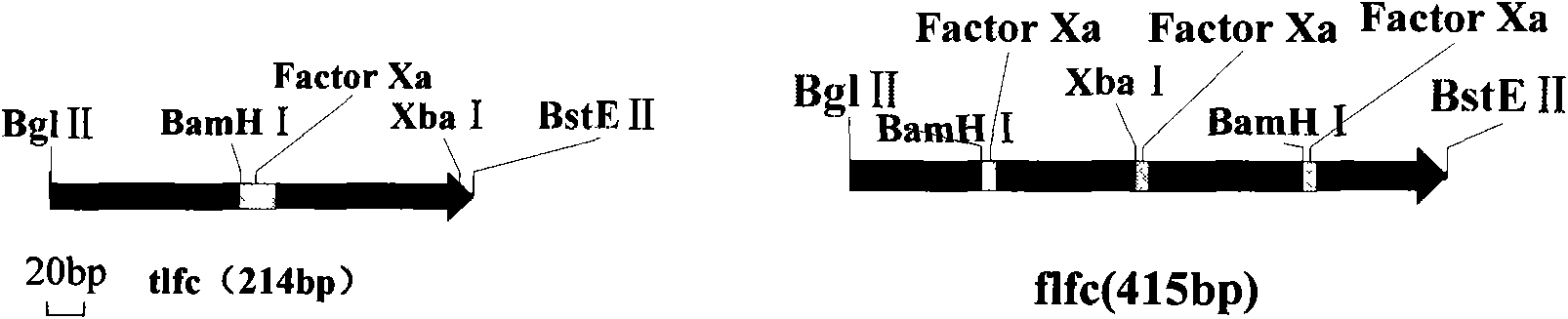Bovine lactoferrin antibacterial peptide fusion protein, coding gene and application thereof
A fusion protein and fusion protein technology are applied to bovine lactoferrin antibacterial peptide fusion protein and its encoding gene and application fields, and can solve the problems of few lactoferrin antibacterial peptide genes, unable to achieve production and application, unsatisfactory expression and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1, two copies of the lactoferrin antimicrobial peptide gene are connected in series
[0054] 1. PCR amplification of lactoferrin antimicrobial peptide gene
[0055] Two long single-stranded DNAs were synthesized by Shanghai Shenyou,
[0056] First chain:
[0057] 5'ACGGGAGCTCAGATCTTCAACAATGGAGTGGTTCAAGTGCCGCCGCTGGCAGTGGCGCATGAAGAAGCTGGGCGCCCCCA 3'
[0058] Second chain:
[0059] 5'AGCTGGTCACCTCTAGATCAGAAGGCGCGGCGCACGCAGGTGATGCTGGGGGCGCCCCAGCTTCTTCATGCGCCACTGCCA 3'
[0060] The two chains are annealed and filled to form a double-chain bovine lactoferrin antimicrobial peptide coding sequence.
[0061] The annealing and filling conditions are: 50uM 1ul of the first strand, 50uM 1ul of the second strand, 0.5ul of Taq DNA polymerase, 2μl of dNTP mixture, 5μl of 10×PCR buffer, MgCl 2 (25uM) 3ul, add water to a final volume of 50ul.
[0062] The above reactant was placed in a 94°C hot water bath, the temperature of the hot water was gradually lowered to 65°C, ...
Embodiment 2
[0077] Example 2, Preparation of the tandem fragment flfc of four lactoferrin antimicrobial peptide gene copies
[0078] 1. PCR amplification of tlfc
[0079] The pGEM-T Easy / tlfc plasmid was used as a template, tlfc1 was amplified with tlfc1SP and tlfc1ASP as a primer pair, and tlfc2 was amplified with tlfc2SP and tlfc2ASP as a primer pair.
[0080] The primer sequences are as follows:
[0081] tlfc1SP 5′ CGGGAGCTCAGATCTTCAACAATGGAGTGGTT 3′;
[0082] tlfc1ASP 5'TCACCTCTAGACCAGAAGGCGCGGCG 3';
[0083] tlfc2SP 5'AGTCTAGAATTGAAGGAAGGGAGTGGTTCAAGT 3';
[0084] tlfc2ASP 5'GGTGGTCACCAGATCAGAAGGCGC 3'.
[0085] The PCR amplification reaction system and reaction procedure were the same as in Example 1.
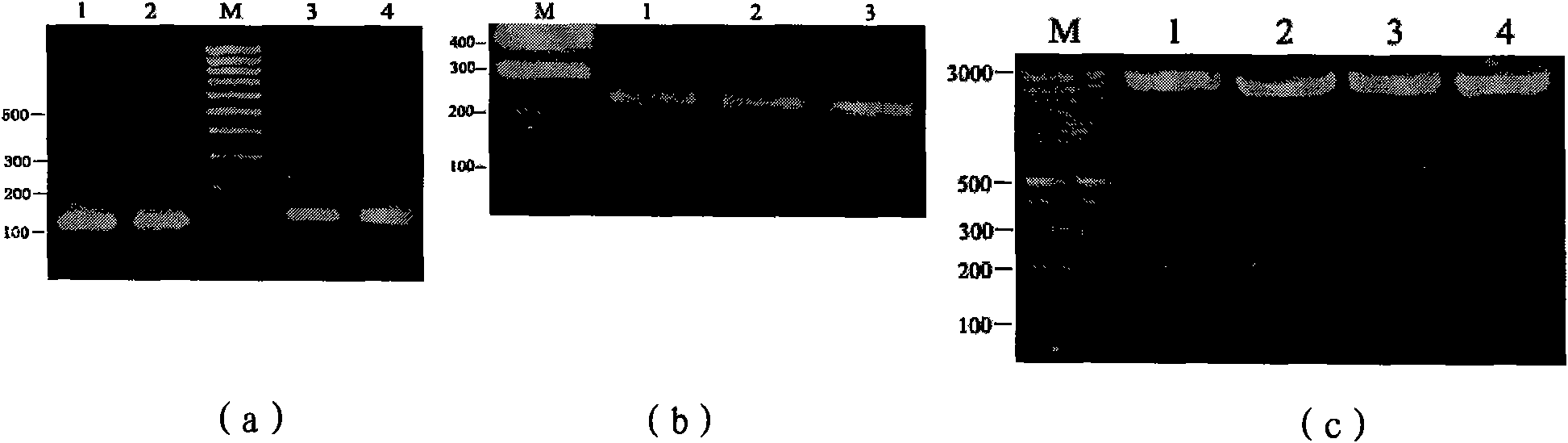
[0086] PCR products were subjected to agarose gel electrophoresis, such as Figure 4 As shown in a, the PCR products of tlfc1 and tlfc2 were recovered, and tlfc1 and tlfc2 were respectively cloned into the pGEM-T Easy vector to construct recombinant plasmids, which were named p...
Embodiment 3
[0090] Embodiment 3, the acquisition of pCAMBIA3301 / tlfc and pCAMBIA3301 / flfc tobacco
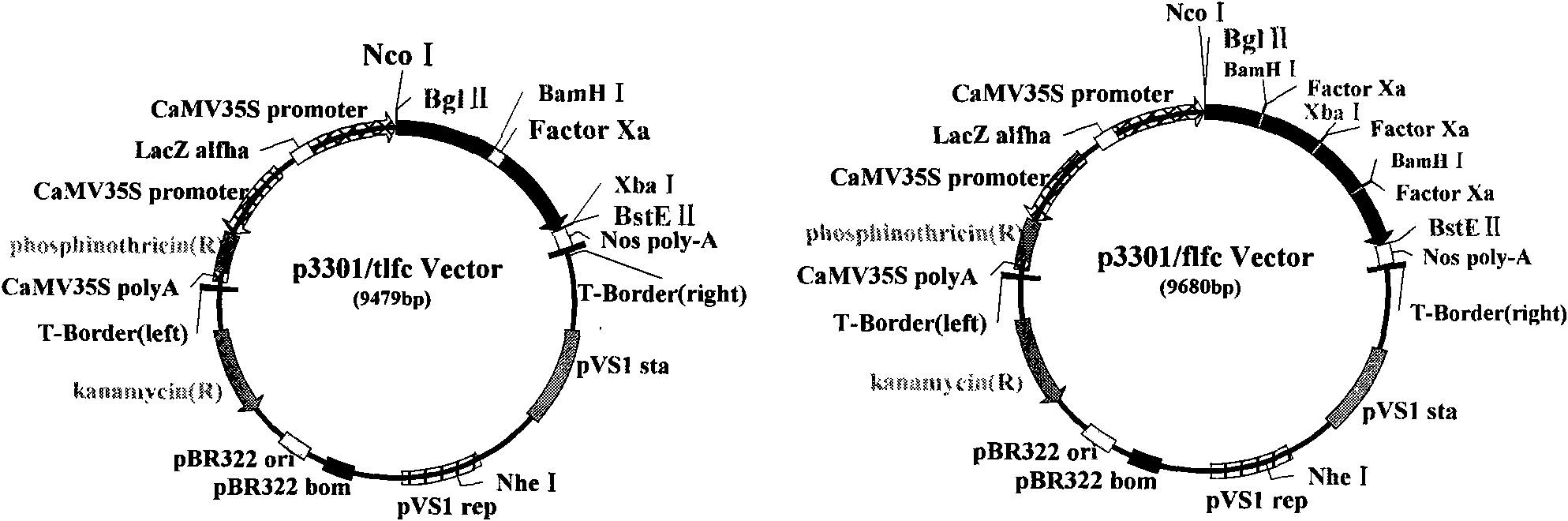
[0091] 1) Construction of plant expression vector pCAMBIA3301 / tlfc and pCAMBIA3301 / flfc
[0092] Digest pGEM-T Easy / tlfc and pGEM-T Easy / flfc plasmids and pCAMBIA3301 plasmids with Bgl II and BstE II respectively, and recover the required fragments, the sizes are 214bp, 415bp and 9.3Kb respectively, and the 214bp fragment of tlfc will be recovered and the 415bp fragment of flfc were respectively ligated with the recovered 9.3Kb fragment of pCAMBIA3301, and the T4 DNA ligase ligation reaction was performed for 4 hours (16°C water bath). Select positive clones, use lfc1SP and lfc2ASP, tlfc1SP and tlfc2ASP as primers for bacterial liquid PCR detection, select bacterial liquid PCR to identify the correct plasmid, and use multi-combination endonucleases for enzyme digestion identification. The results of PCR detection and enzyme digestion identification were as follows: Figure 5 as shown, F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com