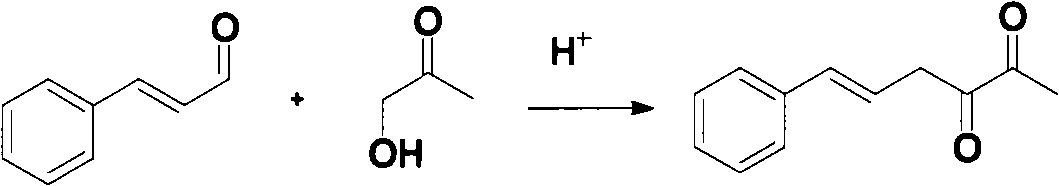Novel flavor compound and preparation method, application and composition thereof
A technology of compounds and compositions, applied in the field of new fragrance compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
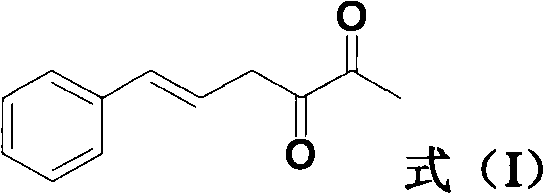
[0024] Example 1: Synthesis of 3-hydroxy-6-phenyl-3,5-hexadien-2-one
[0025] Mix 100g of cinnamaldehyde and 286g of hydroxyacetone at room temperature to obtain a mixture of cinnamaldehyde and hydroxyacetone; add 63g of 15% hydrochloric acid into the reaction flask, raise the temperature to 50°C, and then add the above cinnamon dropwise under stirring at a constant rate within 2h A mixture of aldehyde and hydroxyacetone, keep it for 2 hours after dripping;
[0026] Then, 110 g of water was added to the reaction solution, the temperature was raised to 82° C. and the reaction was continued for 2 h; after the reaction, the product was filtered while hot and recrystallized with ethyl acetate to obtain 39.4 g of white crystals. After testing, its melting point is 127.8-128.1°C; the yield is 27.7%.
[0027] 1 H NMR(CDCl 3 , 400MHz): d 2.45 (s, 3H), 6.39 (d, J = 11.2 Hz, 1H), 6.84 (d, J = 12 Hz, 1H), 6.86 (s, 1H), 7.28-7.34 (m, 2H) , 7.35-7.39 (m, 1H), 7.52-7.54 (m, 2H) ppm;
[0028] 13 C...
Embodiment 2
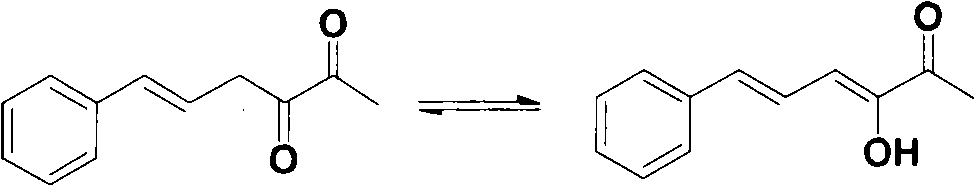
[0030] Example 2: Olfactory evaluation of pure 3-hydroxy-6-phenyl-3,5-hexadien-2-one
[0031] The olfactory evaluation of pure 3-hydroxy-6-phenyl-3,5-hexadien-2-one was completed by an evaluation team composed of several professionals. The compound has a pure and herb-like aroma, with tobacco and bean aromas aroma.
Embodiment 3
[0032] Example 3: The olfactory evaluation of 3-hydroxy-6-phenyl-3,5-hexadien-2-one in the composition
[0033] Component
(%weight ratio)
(%weight ratio)
(%weight ratio)
5%
2%
2.5%
[0034] Squalane
50%
40%
60%
Glyceryl triisocaprylate
20%
25%
20%
20%
23%
17.5%
3-hydroxy-6-phenyl-3,5-
Hexadiene-2-one
5%
10%
0%
Total
100%
100%
100%
[0035] Squalane, glyceryl triisocaprylate and hydroxyethyl cellulose can all be purchased directly from the market.
[0036] Test group 1 and 2 had a light herb-like aroma with tobacco and bean aroma, while test group 3 had no such aroma. In addition, the evaluation team conducted T test on the composition of test group 1, test group 2 and test group 3 in an open environment. 0h , T 72 , T 288h The olfactory evaluation results show that the intensity of the fragrance will decrease with time, but the natu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com