Composition and microsphere for controlled-release of exendin, and method of preparing the same
一种组合物、微球的技术,应用在药物组合、非有效成分的医用配制品、含有效成分的医用配制品等方向,能够解决生物利用度变低、生物利用度不足、不能被应用等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0221] Preparation of microspheres containing Exenatide-4 by spray drying
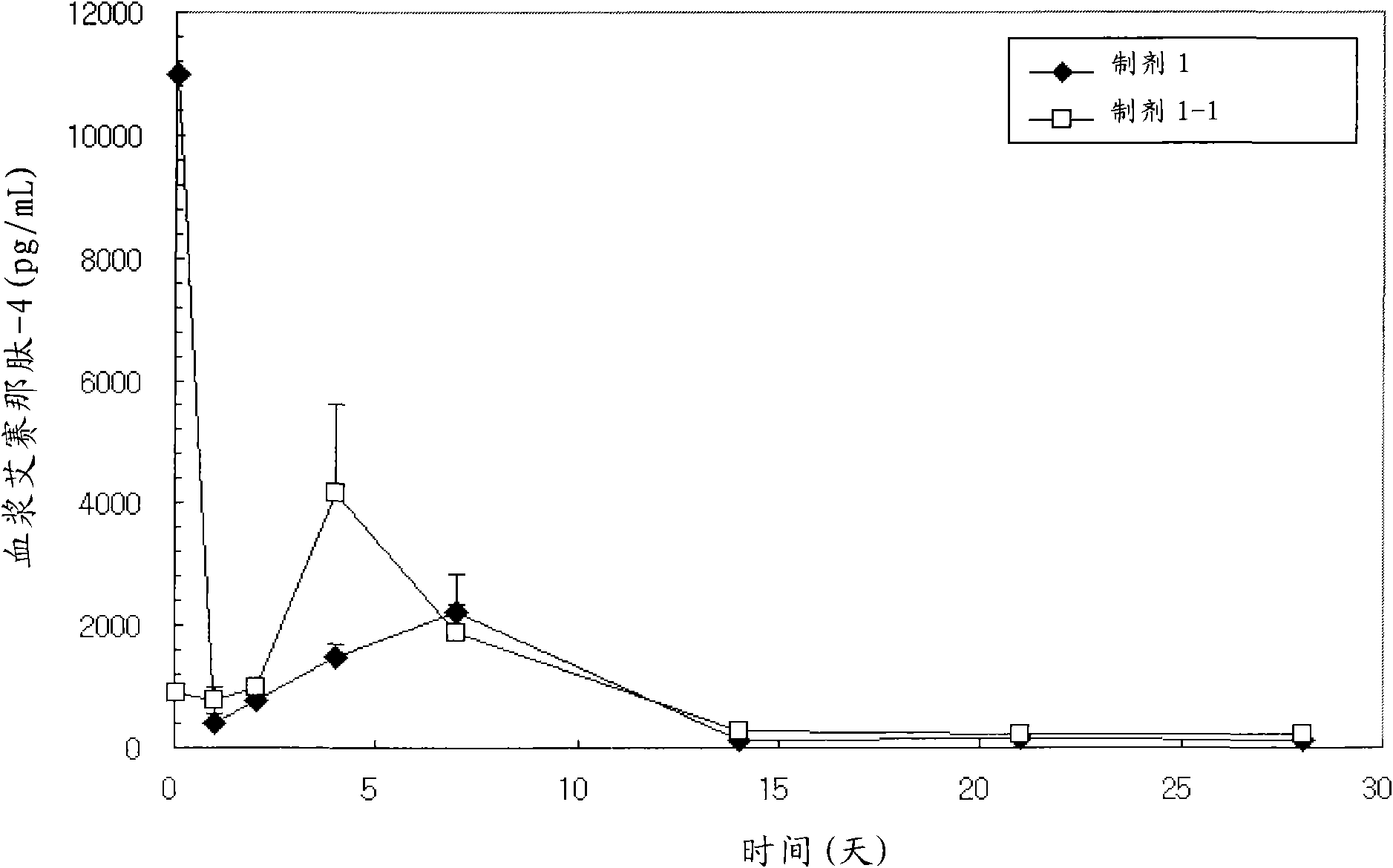
[0222] 4.850 g of biodegradable polymer RG502H (Lot No. 1009848, IV=0.19 dL / g) and 0.150 g of Exenatide-4 (Polypeptide Laboratories, USA) were uniformly dissolved in 97 mL of glacial acetic acid. The solution prepared using a piston pump at a flow rate of 1.5 mL / min was charged into a spray dryer (SODEVA, France) with an ultrasonic nozzle (Sono-tek, 120 kHz) while supplying dry air at 180 °C to prepare Microspheres. The prepared microspheres were suspended in 0.5M lysine aqueous solution (preparation 1-1), suspended in 0.01M lysine aqueous solution (preparation 1-2), suspended in 0.1M histidine aqueous solution (preparation 1-3) and suspended in 0.5M arginine aqueous solution (preparation 1-4), wherein the solution contains 1% (W / V) polyvinyl alcohol (polyvinyl alcohol , Gohsenol, EG-50) as a protective colloid, collected after stirring for 3 hours, washed with distilled water and then lyophilized. ...
Embodiment 2
[0223] depends on the effect of the composition of different polymers
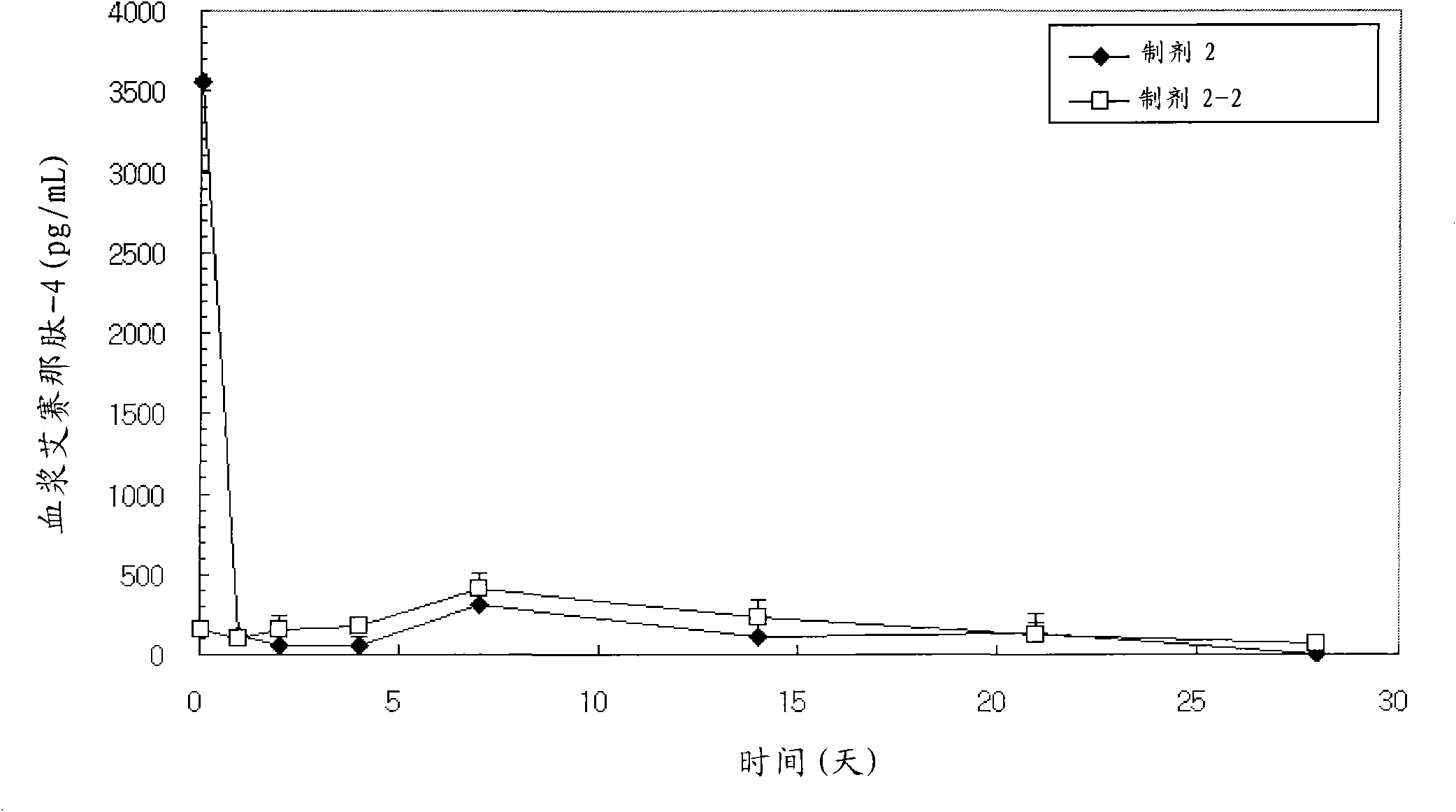
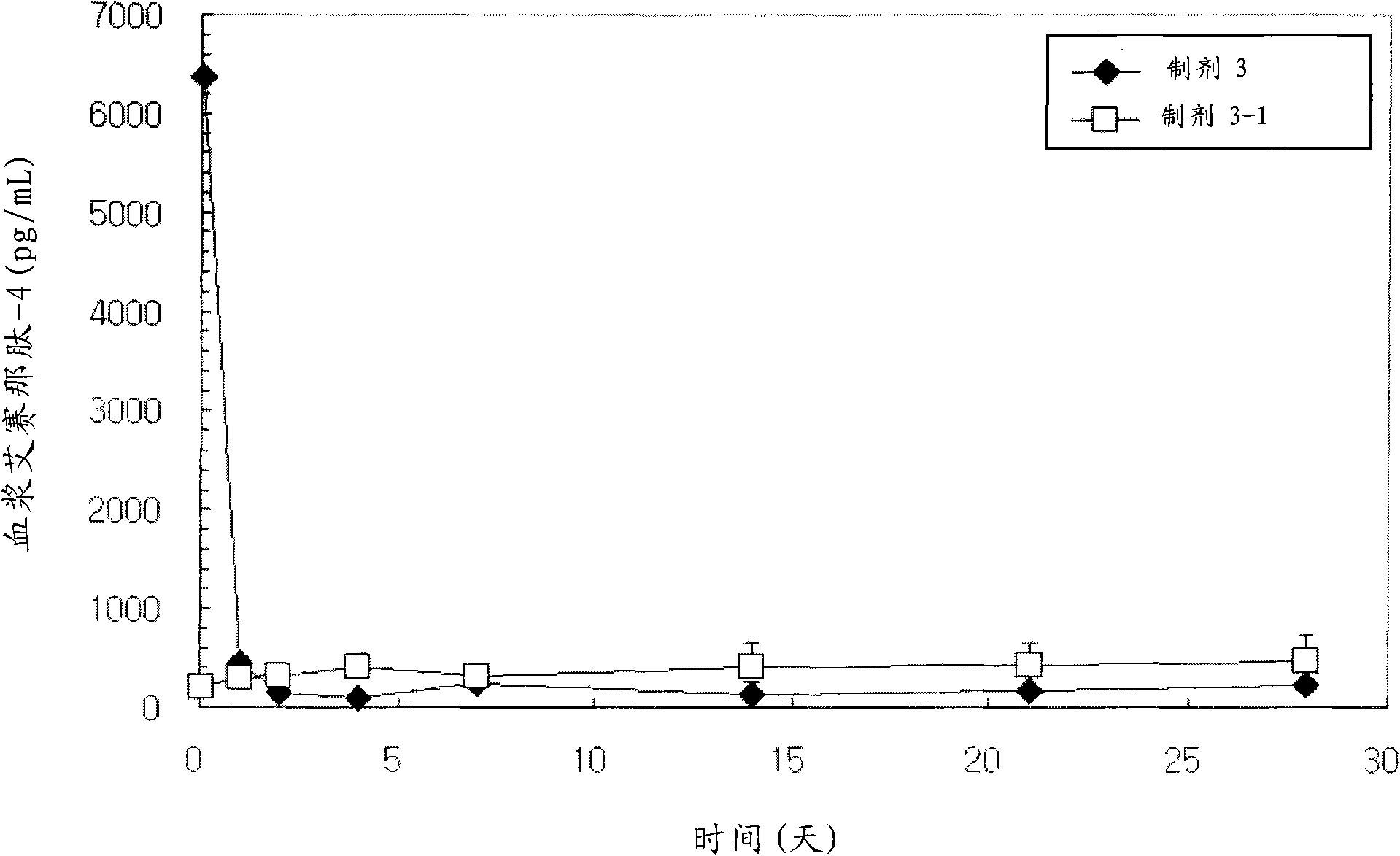
[0224] Exenatide-4-containing microspheres were prepared according to the same method as described in Example 1, the only difference being that the polymers used as biodegradable polymers were: RG503H (Lot No.1006370, IV=0.38 dL / g, formulations 2, 2-1 and 2-2), equal amounts of RG502H and RG503H (Lot No.1009848: Lot No.1006370=1:1, IV=0.29dL / g, formulations 3 and 3-1) Mixture, RG504H (Lot No. 1016605, IV = 0.51 dL / g, formulations 4 and 4-1), 5050DL2A (Lot No. LP-207, IV = 0.18 dL / g, formulations 5 and 5-1) and 5050DL 4A (Lot No. LP-206, IV=0.46 dL / g, formulations 6 and 6-1).
experiment Embodiment 1-1
[0225] Test the effect of microsphere coating layer
[0226] The content of Exenatide in the microspheres prepared in Examples 1 and 2 was quantitatively measured using the following method. Exenatide-4 (Polypeptide Laboratories, USA) was dissolved in DMSO (dimethyl sulfoxide), and diluted with DMSO to a concentration of 2, 5, and 10 μg / mL, respectively, the solution was used as a standard solution, and used A fluorescence photometer (Cary Eclipse, Varian, USA) was used for fluorescence measurement at Ex 280nm and Em 350nm to obtain a measurement curve. The prepared microspheres were dissolved in DMSO to a concentration of 150 μg / mL, and then the fluorescence measurement was also performed, and the exenatide content in the microspheres was measured by extrapolation from the above measurement curve.
[0227] The content of the coating material used in the composition of the present invention, specifically, the content of lysine, arginine, histidine, etc. contained on the surf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com