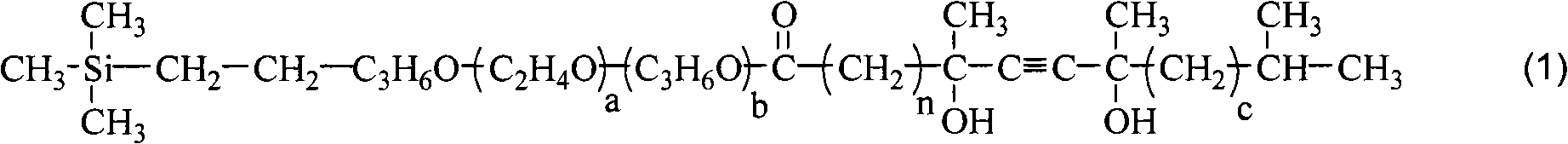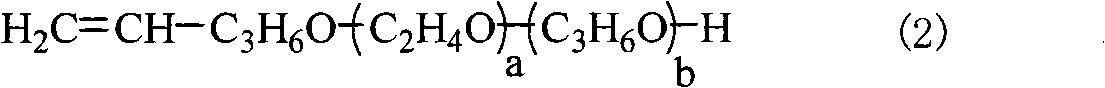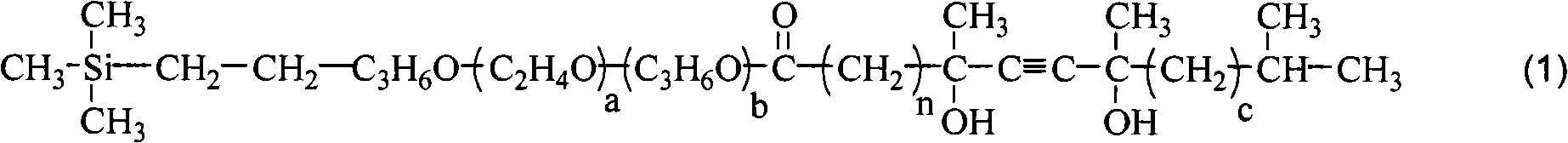Disilane surfactant, preparation thereof and use thereof
A technology of surfactant and silyne, which is applied in the field of agricultural chemicals, can solve the problems of undiscovered reports on the preparation and application of silyne surfactants, failure to meet requirements, easy hydrolysis of silicone surfactants, etc., and achieve good resistance The effects of rain scourability, excellent defoaming performance, and excellent pore permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0045] The synthesis of example 1 silyne surfactant 1#
[0046] 1.1. Synthesis of vinyl-terminated acetoacetate (code 1#A)
[0047]Add 496 grams (1 mole) of terminal vinyl ether (a=8, b=1), 195 grams (1.5 moles) of acetoacetic acid in the four-neck flask equipped with stirring, thermometer, rectifying tower device and nitrogen device Ethyl ester and 5 grams of polyferric sulfate catalyst are heated up to 80-120°C, and the by-product ethanol is continuously distilled from the top of the tower. After 6 hours the reaction was complete. The polyferric sulfate catalyst was separated by filtration while hot. The filtrate was concentrated in vacuo to remove unreacted ethyl acetoacetate and residual ethanol to obtain 574.2 g of vinyl-terminated acetoacetate with a yield of 99%, a colorless liquid, and a purity of 98.6%.
[0048] 1.2. Synthesis of vinyl-terminated acetylenic diol (code 1#B)
[0049] Add 500 milliliters of MTBE (methyl tert-butyl ether), 100 grams of potassium hydro...
example 2
[0052] The synthesis of example 2 silyne surfactant 2#
[0053] 2.1. Synthesis of vinyl-terminated acetoacetate (code 1#A)
[0054] Feeding and operation are the same as Example 1.1.
[0055] 2.2. Synthesis of vinyl-terminated alkyne diol (code 2#B)
[0056] Replace 4-methyl-2-pentanone with 21.5 g (0.25 mole) of 3-methyl-2-butanone, and the rest of the addition and operation are the same as in Example 1.2. 143.6 g of ethylene-terminated acetylenic diol was obtained, with a yield of 83%, as a light yellow viscous liquid, with a purity of 97%.
[0057] 2.3. Synthesis of silyne surfactant 2#
[0058] Add 138.4 grams (0.2 moles) of ethylene-terminated acetylenic diol (code name 2#B) prepared in Example 2.2, and the rest of the addition and operation are the same as Example 1.3. Obtain silyne surfactant 2#, light yellow viscous liquid, weighing 151.7 grams, yield 99%, purity 96%.
example 3
[0059] The synthesis of example 3 silikyne surfactant 3#
[0060] 3.1. Synthesis of vinyl-terminated acetoacetate (code 3#A)
[0061] Add 438 g (1 mole) of terminal vinyl ether (a=8, b=0), 216 g (1.5 mole) of ethyl levulinate, and the rest of the addition and operation are the same as Example 1.1. 505 g of terminal vinyl ether levulinate was obtained, with a yield of 96%, as a colorless liquid with a purity of 99%.
[0062] 3.2. Synthesis of vinyl-terminated alkyne diol (code 3#B)
[0063] 131.5 grams (0.25 moles) of terminal vinyl ether levulinate (code name 3#A) prepared in 3.1 as an example of the dripping material, and the rest of the feeding and operation are the same as in Example 1.2. 140.2 g of ethylene-terminated acetylenic diol was obtained, with a yield of 86%, as a light yellow viscous liquid, with a purity of 98%.
[0064] 3.3, silyne surfactant 3#
[0065] Add 130.4 g (0.2 moles) of ethylene-terminated acetylenic diol (code 3#B) prepared in Example 3.2, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com