Recombinant expression vector containing Schistosoma japonicum gene and its application
An expression vector, the technology of schistosomiasis thioredoxin, applied in the field of bioengineering, can solve the problems that there are no research reports on thioredoxin peroxidase II of Schistosoma japonicum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Construction of Schistosoma japonicum tpII gene coding sequence prokaryotic expression vector
[0032] Find the sequence of Schistosoma japonicum tpII gene in Genebank to design primers, analyze its sequence characteristics with DNAstar, and find its largest open reading frame (SEQ ID NO: 2), that is, the sequence part of its coding protein, and design primers based on this part of the sequence as follows:
[0033] Sense: 5'-GGC GGATCC ATGAAGTGTTTAAATTCG-3' (SEQ ID NO: 3),
[0034] Anti-sense: 5'-GGC CTCGAG GTTTACAGAGGAAAAGTACG-3' (SEQ ID NO: 4),
[0035] The restriction endonucleases BamH I and Xho I restriction sites (underlined parts) were respectively introduced. The TpII open reading frame was amplified by PCR using the cDNA library of female Schistosoma japonicum as a template. The prokaryotic expression vector pET28a(+) used is a high-efficiency expression vector expressing 6×His tags. The vector is designed with multiple cloning restriction site...
Embodiment 2
[0038] Example 2 Induced Expression of Schistosoma japonicum tpII Gene Prokaryotic Expression Vector
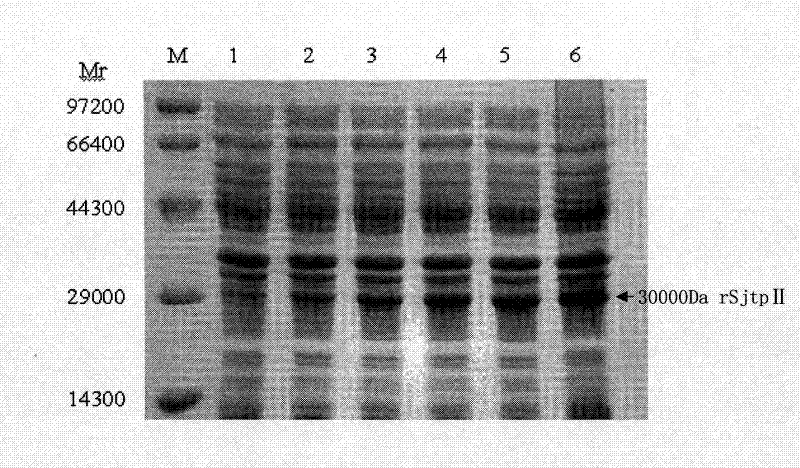
[0039]The plasmid SjtpII-pET28a(+) of the positive clone with correct sequencing was transformed into Escherichia coli BL21(DE3) competent cells, spread on the LB plate containing kanamycin, picked a single colony, and inoculated in In the liquid LB medium, after reaching the logarithmic growth phase, add isopropyl-β-D-thiogalactopyranoside (IPTG, the final concentration is 1mM / L) to induce expression, collect before induction and 1h after induction , 2h, 4h, 6h and 8h of the bacterial solution were analyzed and verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE electrophoresis, 5% stacking gel 80V voltage, 12% separating gel 120V voltage).
[0040] The result is as figure 2 shown in figure 2 Middle, M: protein marker; 1: Uninduced bacterial fluid; 2: 1 h after induction; 3: 2 h after induction; 4: 4 h after induction; 5: 6 h after induction; ...
Embodiment 3
[0041] Embodiment 3 Purification of prokaryotic expression vector expression product
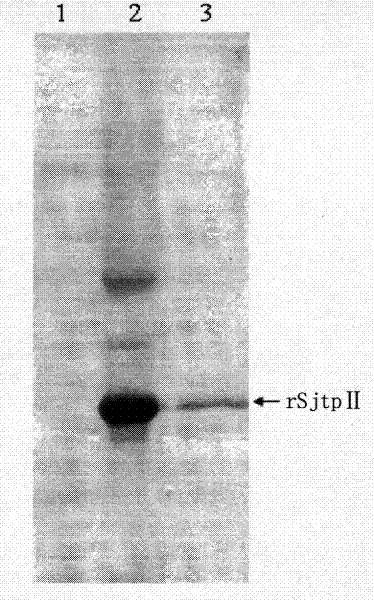
[0042] Induce a small amount of recombinant bacteria (100ml), centrifuge at 4°C, 4000rpm for 15min, collect the bacterial pellet, wash with PBS, add 1× binding buffer (binding buffer) to resuspend the bacterial cells, freeze and thaw 3 times, and then ultrasonically disrupt cell. The sonicated suspension was centrifuged at 4° C., 12,000 rpm for 30 min, and the supernatant and precipitate were collected to identify its expression form. Depend on image 3 It can be seen that the expression product of the recombinant plasmid appears in the precipitate after ultrasonication, indicating that its expression form is inclusion body. exist image 3 Middle, 1: Supernatant after sonication; 2: Precipitation after sonication; 3: Protein after purification.
[0043] The fusion protein can be purified by utilizing the affinity of histidine and nickel ions in the recombinantly expressed fusion protein,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com