Method for dry preparation of barium fluorosilicate and barium fluoride
A technology of barium fluorosilicate and barium fluoride, which is applied in the field of barium fluoride prepared by dry method and co-produced intermediate product barium fluorosilicate, which can solve the problems affecting the cost of barium fluoride, the increase of hydrofluoric acid price, and the barium fluoride Fine particles and other problems, to achieve the effect of easy popularization and application, low cost, and ease the pressure of environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
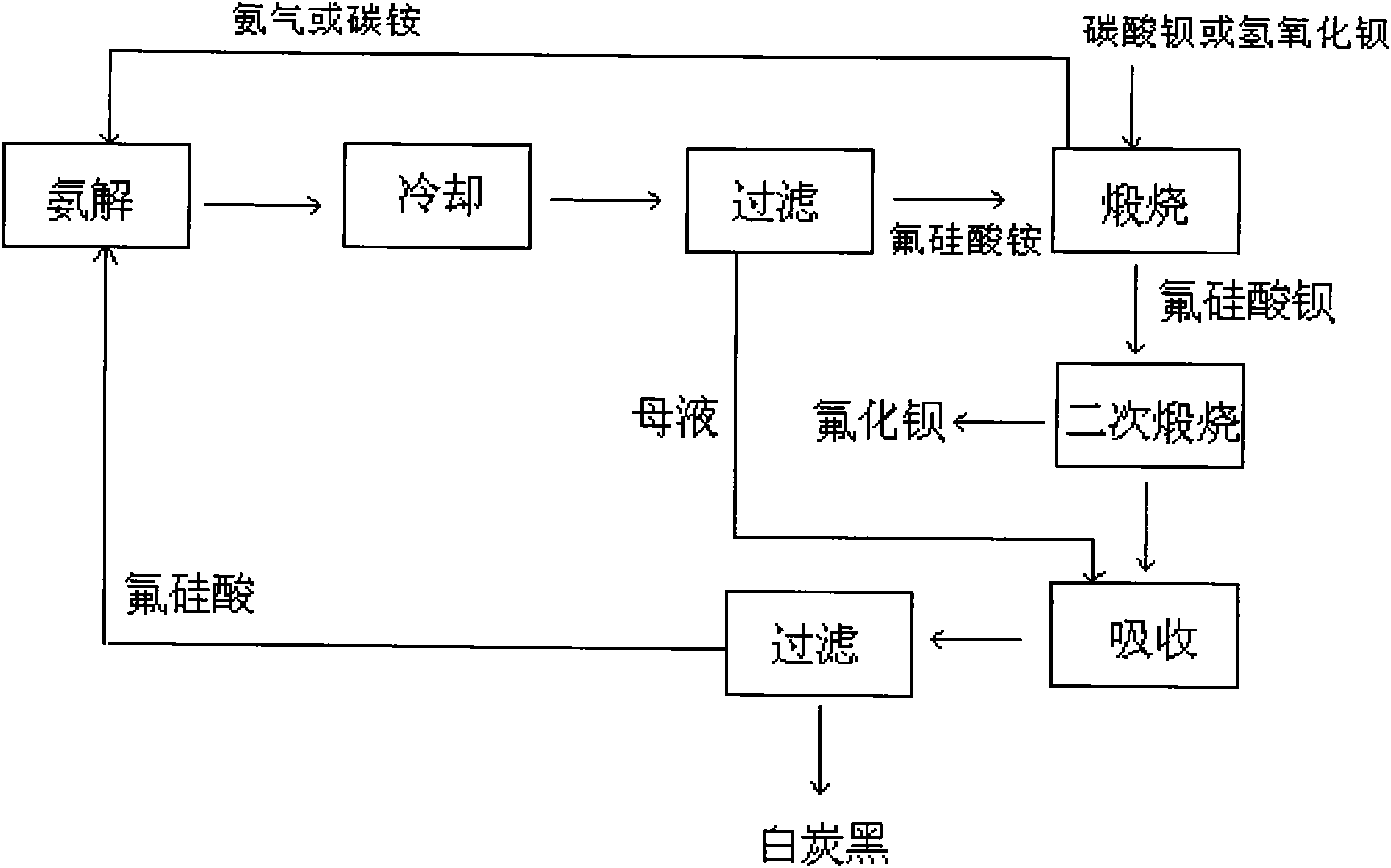
[0043] The method for preparing barium fluoride and barium fluorosilicate by the dry method of the present embodiment is to use ammonium fluorosilicate and barium carbonate as the main raw materials on the basis of fluorosilicic acid and ammonium ammonia solution, and specifically includes the following steps:
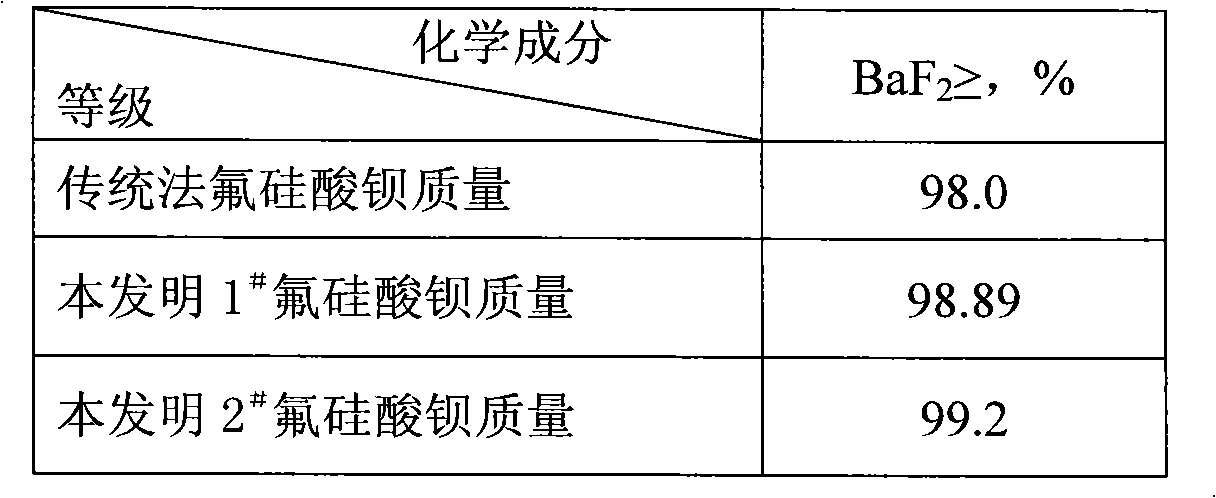
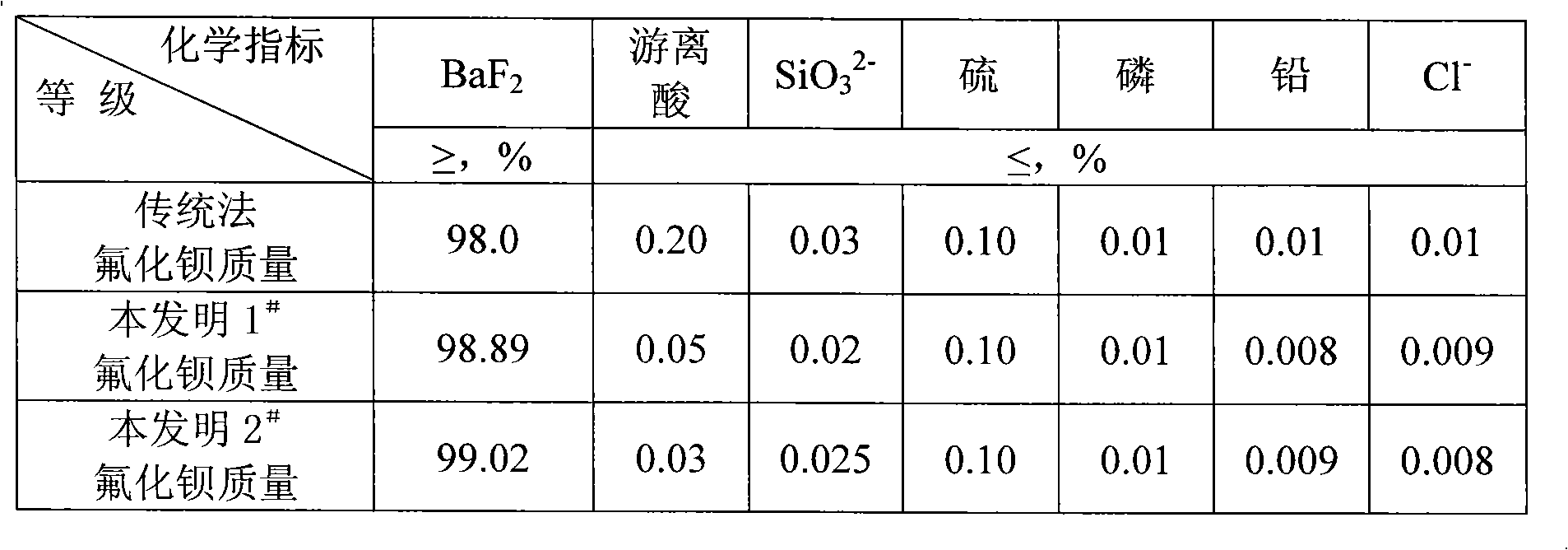
[0044]Add 280ml of 10mol / l ammonia water to 500g of 40% fluosilicic acid solution to carry out ammonolysis, cool and filter to obtain 235g of ammonium fluosilicate; take the prepared 235g of ammonium fluosilicate and mix with 262g of 98% barium carbonate Evenly, decompose at a temperature of 180°C for 4 hours to produce 370g of barium fluorosilicate solid and ammonium bicarbonate; the ammonium bicarbonate generated during the decomposition is passed into the fluorosilicic acid solution, and ammonium fluorosilicate is obtained again, and the rest of the gas is discharged Empty; the generated barium fluorosilicate solid was heated to 550°C and calcined for 8 hours to gene...
Embodiment 2
[0046] The method for preparing barium fluoride and barium fluorosilicate by the dry method of this embodiment is based on fluorosilicic acid and ammonium ammonia solution, using ammonium fluorosilicate and barium hydroxide as the main raw materials, specifically comprising the following steps:
[0047] Add 280ml of 10mol / l ammonia water to 500g of 40% fluosilicic acid solution for ammonolysis, cool and filter 235g of ammonium fluosilicate; mix 235g of ammonium fluosilicate with 230g of barium hydroxide, Decompose at lower temperature for 4 hours to generate 370g of barium fluorosilicate solid and ammonia gas; the ammonia gas generated during the decomposition is passed into the fluorosilicate solution, and the ammonium fluorosilicate is re-ammonolyzed, and the rest of the gas is emptied; the generated fluorosilicate The barium solid is heated to 600°C and calcined for 6 hours to generate barium fluoride solid and silicon tetrafluoride gas; the silicon tetrafluoride gas is abso...
Embodiment 3
[0049] The method for preparing barium fluoride and barium fluorosilicate by the dry method of the present embodiment is to use ammonium fluorosilicate and barium carbonate as main raw materials on the basis of ammonium hydrolysis of fluorosilicic acid and ammonium bicarbonate, and specifically includes the following steps:
[0050] Add 125g of ammonium carbonate with an ammonia content of 16.8% to 500g of 15% fluosilicic acid solution to carry out ammonolysis, and cool and filter to obtain 85g of ammonium fluosilicate; Mix 89g of barium carbonate evenly, decompose at 180°C for 3 hours to produce 124g of barium fluorosilicate solid and ammonium bicarbonate; the ammonium bicarbonate produced during the decomposition is passed into the fluorosilicate solution, and ammonium hydrolysis is made again to obtain ammonium fluorosilicate , the rest of the gas is evacuated; the generated barium fluorosilicate solid is heated to 550 ° C for 6 hours to generate barium fluoride solid and si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com