Method for manufacturing neuraminic acid derivatives
A compound and alkyl technology, applied in the field of neuraminic acid derivatives, can solve the problems of expensive silyl protecting groups and low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0498] (4S,5R,6R)-5-acetamide-4-guanidino-6-[(1R,2R)-2-hydroxy-1-methoxy-2-(octanoyloxy)propyl]-5 Synthesis of ,6-Dihydro-4H-pyran-2-carboxylic acid [Compound (Ib)]
[0499] Step A-1: N-acetylneuramate methyl ester
[0500] Trimethyl orthoformate (116.67 g) and methanol (2720 ml) were added to N-acetylneuraminic acid (340.00 g) and suspended. Under stirring at room temperature, concentrated sulfuric acid (8.63 g) was added to the suspension, and the mixture was stirred at 40°C for 3 hours. The solvent was distilled off under reduced pressure until the amount of the solution became about 1530 ml, dibutyl ether (4420 ml) was added to the reaction solution at 30°C, and the reaction solution was stirred at the same temperature for 1 hour. After stirring it for another 1 hour at 0°C, the crystals were filtered. The crystals were washed with a mixture of methanol (170 ml) and dibutyl ether (510 ml) and dried under reduced pressure to obtain the title compound as a white solid (342.1...
Embodiment 2
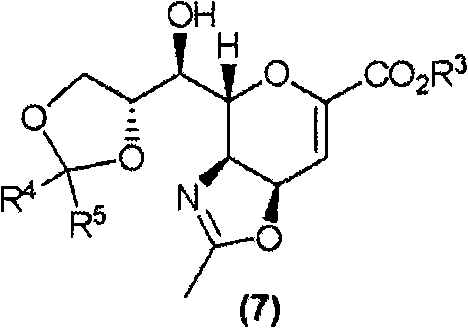
[0554] (3aS,4R,7aR)-4-{(S)-hydroxy[(4R)-2-oxo-1,3dioxolane-4-yl]methyl}-2-methyl-3a,7a -Dihydro-4H-pyrano[3,4-d][1,3]oxazole-6-carboxylic acid methyl ester (Compound (7) [R 4 , R 5 =oxo group]) synthesis
[0555] Step A-1: N-acetylneuraminic acid methyl ester
[0556] Trimethyl orthoformate (5.14 g) and methanol (120 ml) were added to N-acetylneuraminic acid (1) (15.00 g) and suspended. At room temperature, concentrated sulfuric acid (0.38 g) was added while stirring, and the reaction solution was stirred at 40°C for 3 hours. After the completion of the reaction, N,N-dimethylacetamide (15 ml) was added to the reaction solution, and then the solvent was distilled off under reduced pressure until the amount of the solution became about 40 ml. At 20°C, water (7.5ml) and ethyl acetate (150ml) were added to the concentrated solution, the mixture was stirred at 30°C for 0.5 hours, then ethyl acetate (150ml) was added and stirred at the same temperature for another 0.5 hour. After s...
Embodiment 3
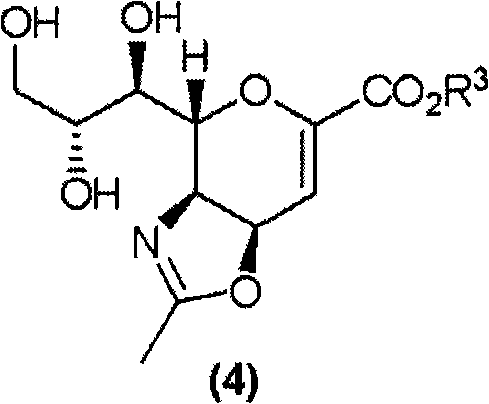
[0564] (4S,5R,6R)-5-acetamide-4-guanidino-6-[(1R,2R)-2,3-dihydroxy-1-methoxypropyl]-5,6-dihydro- 4H-pyran-2-carboxylic acid (compound (13) (R 2 =Methyl]) Synthesis
[0565] Step B-1: (4S, 5R, 6R)-5-acetamide-4-amino-6-{(S)-methoxy[(4R)-2-oxo-1,3-dioxolane- 4-yl]methyl}-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester
[0566] At room temperature, ethyl acetate (40ml), triphenylphosphine (7.79g) and water (1.94g) were added to the compound (10.00g) obtained in step A-6 of Example 1, followed by stirring at 72°C for 2.5 hour. The reaction solution was cooled to room temperature to obtain an ethyl acetate solution of the title compound.
[0567] Step B-2: (4S,5R,6R)-5-acetamide-4-[2,3-bis(tert-butoxycarbonyl)guanidino]-6-{(S)-methoxy[(4R )-2-oxo-1,3-dioxolan-4-yl]methyl}-5,6-dihydro-4H-pyran-2-carboxylic acid methyl ester
[0568] At room temperature, tert-butyl (tert-butoxycarbonyliminopyrazol-1-yl-methyl)carbamate (8.80g) was added to the ethyl acetate solution of the compound o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com