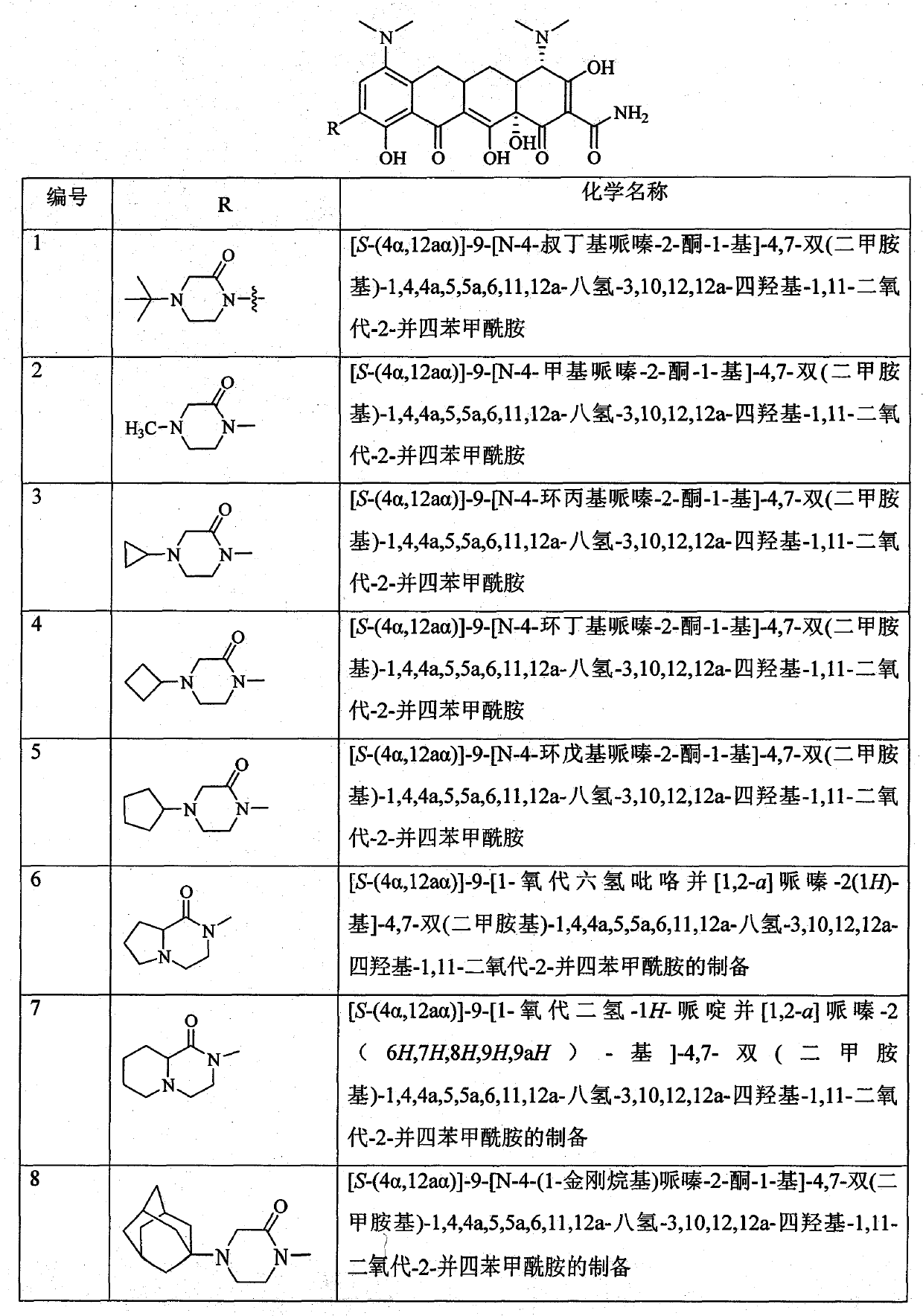
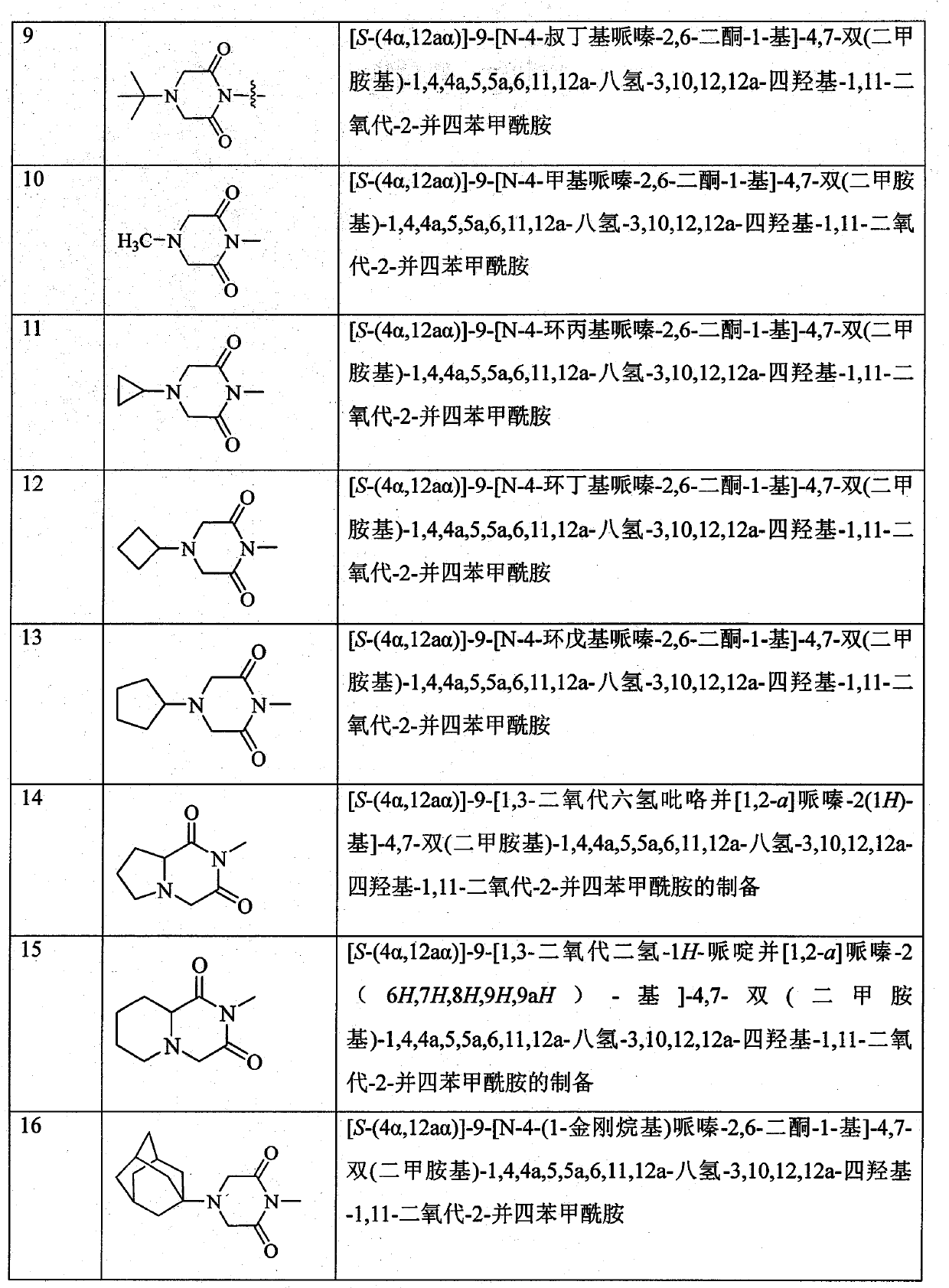
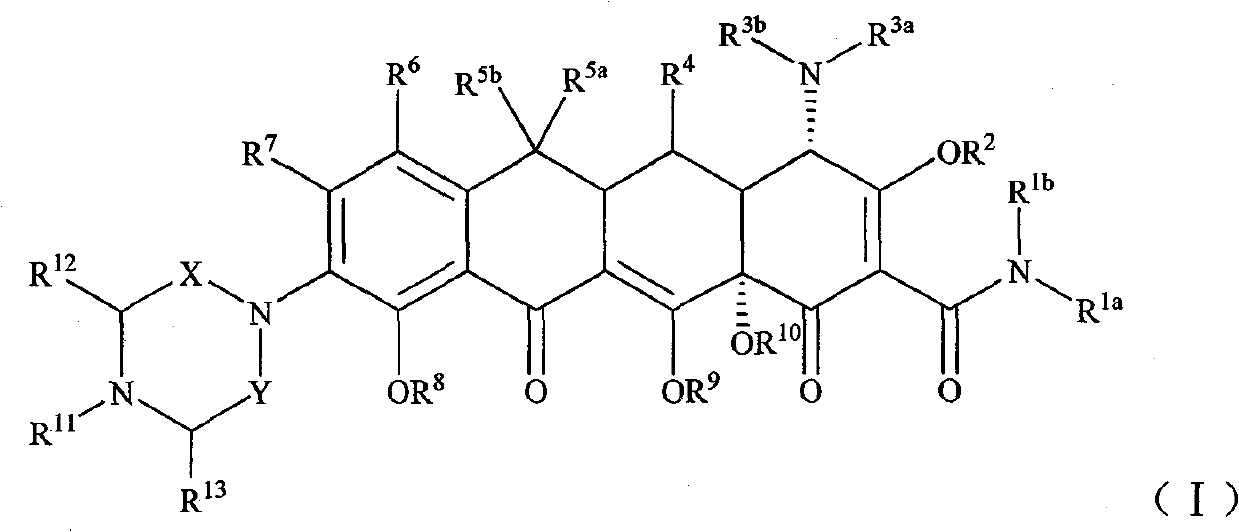
Piperazinone substituted tetracycline derivatives
A compound, cyclopentyl technology, applied in sensitive fields, can solve problems such as unsatisfactory activity of Gram-negative bacteria, inconvenient medication, and pain of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1 [S-(4α, 12aα)]-9-amino-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3, Preparation of 10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide
[0114] Throw 22.8g (50mmol) [S-(4α,12aα)]-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro in the reaction bottle -3,10,12,12a-Tetrahydroxy-1,11-dioxo-2-naphthalene carboxamide dihydrochloride, dissolved in 150ml of concentrated sulfuric acid, stirred and cooled in an ice bath, then added 6.8g sodium nitrate, and the mixture was stirred in an ice bath for 1 h. After the reaction was completed, the mixture was added dropwise to 2000ml of ether, and a solid was precipitated, which was washed with a small amount of ether and dried. Add the solid to 100ml of ethanol, then add 2g of 10% palladium carbon, and stir at room temperature under 2MPa hydrogen pressure for 1.5h. After filtration and concentration under reduced pressure, 800ml of ether was added to the residue under vigorous stirring. Filter a...
Embodiment 2
[0115] Example 2 Preparation of 4-tert-butylmorpholine-2,6-dione
[0116] 1. Preparation of 2,2'-[(tert-butyl)amino]diacetic acid
[0117] Add 4.7g (50mmol) of chloroacetic acid, 40ml of water, 5.6g (100mmol) of KOH, and 1.8g (25mmol) of tert-butylamine to the reaction flask, heat it in a water bath to 70°C, react for 4h and then cool it to room temperature. Neutralize, distill under reduced pressure, filter, adjust the pH value of the filtrate to about 3, and cool to obtain 3.5 g of the product, yield: 75.0%.
[0118] 2, Preparation of 4-tert-butylmorpholine-2,6-dione
[0119] Add 9.5g (50mmol) of 2,2'-[(tert-butyl)amino]diacetic acid and 20ml of acetic anhydride to the reaction flask, heat it in a water bath to 70°C, react for 24h and distill under reduced pressure to obtain 5.3g of the product, yield: 62%.
Embodiment 3
[0120] Example 3 [S-(4α, 12aα)]-9-[N-4-tert-butylpiperazine-2,6-dione-1-yl]-4,7-bis(dimethylamino)- 1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-tetracenecarboxamide (Compound 9) preparation
[0121] Add 100ml of toluene and 8.9g (52mmol) of 4-tert-butylmorpholine-2,6-dione into the dry reaction flask, stir and raise the temperature to above 80°C, slowly add [S-(4α, 12aα)] in batches -9-amino-4,7-bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11 -Dioxo-2-tetracenecarboxamide 14.2g (30mmol), stirred at reflux for 4h. After the reaction is complete, cool to 60°C. Poured into crushed ice with vigorous stirring, a solid precipitated. After filtration, the filter cake was recrystallized with ethanol to obtain 12.7 g of the target compound, yield: 67.8%.
[0122] Molecular formula: C 31 h 39 N 5 o 9 Molecular weight: 625.67 Mass spectrum: (m / e): 626 (M+1)
[0123] Elemental analysis: Theoretical value: C, 59.51%; H, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com