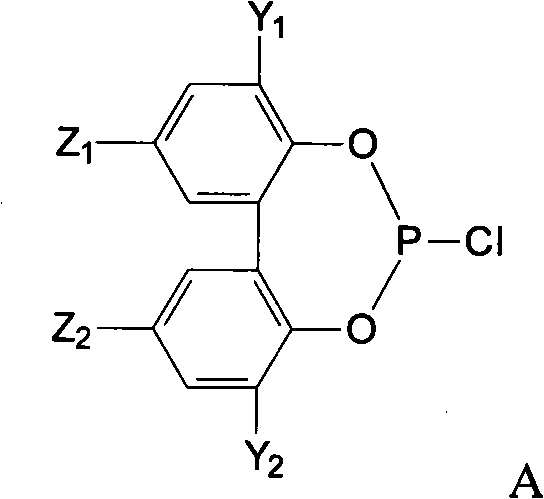
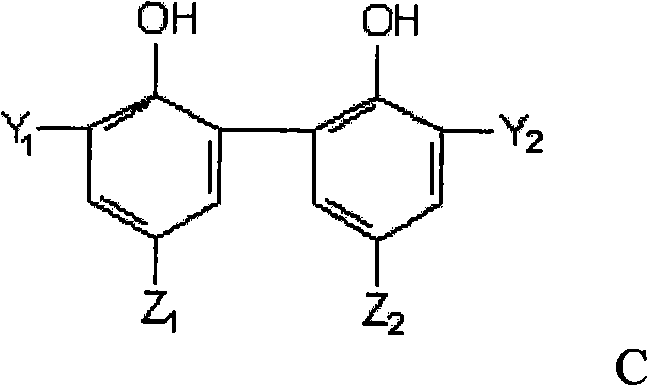
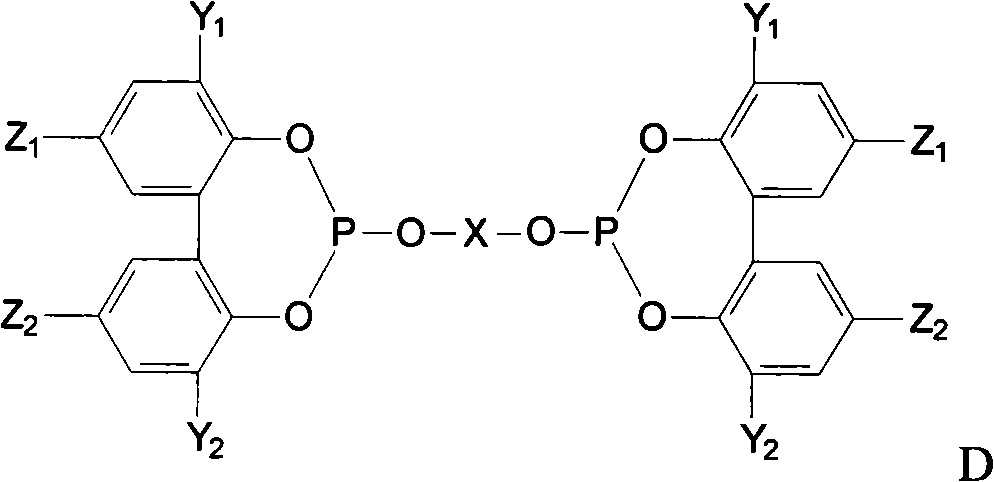
Preparation method of phosphite ester
A technology of phosphite and chlorophosphite, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc. Major problems, to achieve the effect of simplifying the process, reducing the difficulty and increasing the product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Embodiment 1: use dichloromethane as solvent, reaction synthesis phosphite
Embodiment 11
[0083]
[0084] 14g 2,2'-biphenol and 40ml PCl 3 (2,2'-biphenol: PCl 3 The molar ratio is 1:6.1) reacted at 100°C for 4hr, then distilled off excess PCl 3, to obtain 19 g of residue, namely intermediate (2-9). Add 100ml of dichloromethane solvent to the intermediate (2-9) for dilution (the molar ratio of intermediate:dichloromethane is 1:21). Then 2,2'-dihydroxy-3,3'-di-tert-butyl-5,5'-dimethoxy-1,1'-biphenyl solution (2,2'-dihydroxy-3,3 '-Di-tert-butyl-5,5'-dimethoxy-1,1'-biphenyl 13.4g, triethylamine 50ml, dichloromethane 20ml, 2,2'-dihydroxy-3,3'- Di-tert-butyl-5,5'-dimethoxy-1,1'-biphenyl:triethylamine:dichloromethane molar ratio is 1:16.7:8.3) slowly drop into intermediate (2-9) solution (the molar ratio of intermediate to 2,2'-dihydroxy-3,3'-di-tert-butyl-5,5'-dimethoxy-1,1'-biphenyl is 2:1) , control the temperature at about -35°C, rise to room temperature after the dropwise addition, and stir for 12hr. The reactants still remain in solution state, add deionize...
Embodiment 12
[0086]
[0087] 14g 2,2'-biphenol and 40ml PCl 3 (2,2'-biphenol: PCl 3 The molar ratio is 1:6.1) reacted at 100°C for 4hr, then distilled off excess PCl 3 , to obtain 19 g of residue, namely intermediate (2-9). Add 80 ml of dichloromethane solvent to the intermediate (2-9) for dilution (the molar ratio of intermediate:dichloromethane is 1:17). Then biphenol solution (2,2'-biphenol 7g, triethylamine 20ml, dichloromethane 10ml, 2,2'-biphenol: triethylamine: the molar ratio of dichloromethane is 1: 6.7: 4.1) Slowly drop into the solution of intermediate (2-9) (the molar ratio of intermediate to 2,2'-biphenol is 2:1), control the temperature at about -20°C, rise to room temperature after the addition, and stir for 12hr . The reactants still remain in solution state, add deionized water to stir, let stand to separate layers, and take the lower organic phase. Heat to distill off the dichloromethane in the organic phase, and then recrystallize in hexane to obtain the product....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com