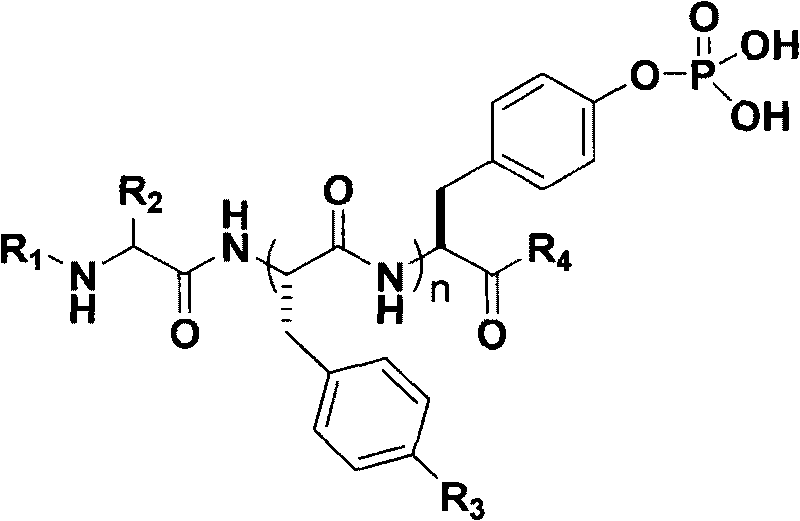
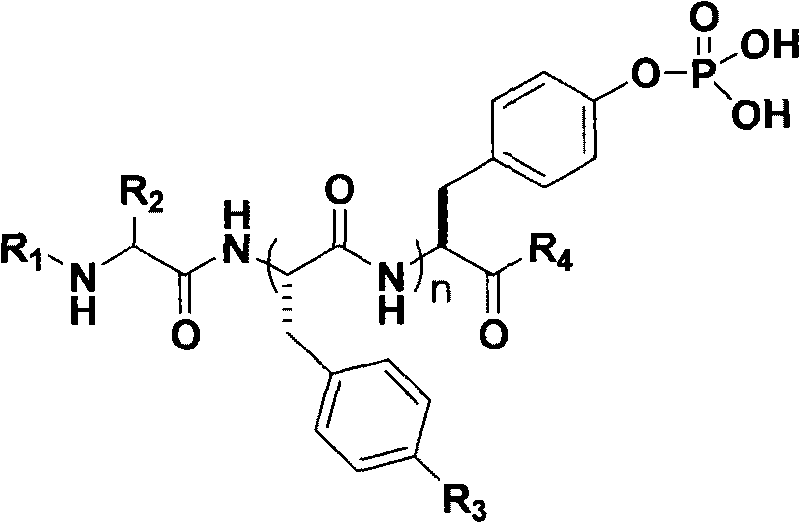
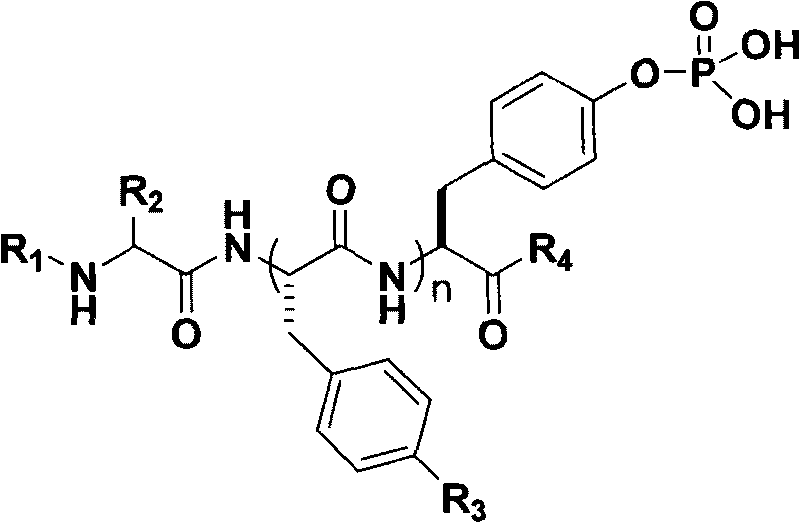
Polypeptide derivatives for generating stable micro-molecular hydrogel
A technology of small molecule water and peptide derivatives, applied in the field of peptide derivatives, can solve problems such as peptides that have not been seen yet, and achieve high application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] The synthetic method step of the polypeptide for generating stable small molecule hydrogel of the present invention is as follows:
[0013]
[0014] Wherein, DIPEA is N, N-diisopropylethylamine, Acetone is acetone, I 2 is iodine, triethyl phosphite is triethyl phosphite, DCM is dichloromethane, pyridine is pyridine, TFA is trifluoroacetic acid, spps is the English abbreviation of "polypeptide solid-phase synthesis", TMSBr is trimethylbromosilane, and MeOH is Methanol.
[0015] Step 1, Fmoc-L-Tyr-O t Synthesis of Bu
[0016] 10mmol L-tert-butyl tyrosine and 10 molecules of N,N-diisopropylethylamine are dissolved in 75ml of acetone, and the acetone solution of 75ml of Fmoc-OSu containing 9.8mmol is added under stirring conditions, Stir at room temperature for 12 hours, then separate with silica gel column to obtain 4.4 g of product Fmoc-L-Tyr-O t Bu (i.e. the compound 3 in the above-mentioned synthetic method step), its productive rate is 95.8%, 1 H NMR (300MHz, D...
Embodiment 2
[0028] Synthesis of the polypeptide compound Nap-GFFY(p)-OMe shown in the following structural formula (I).
[0029]
[0030] The first step to the third step are all the same as in Example 1.
[0031] In the fourth step, the compound Nap-GFFY(PO(OFt) 2 )-OMe (that is, in the general formula of compound 6 of Example 1, R 1 for group, R 2 for H, R3 for the synthesis of H)
[0032] Adopt the method for Fmoc solid phase synthesis, step is:
[0033] Step 1, weigh 0.5mmol 2-chlorotrityl chloride resin in a solid-phase synthesizer, add 2.5mL of anhydrous dichloromethane, and feed nitrogen for 5min to make the 2-chlorotrityl chloride resin fully swelling;
[0034] In the 2nd step, dichloromethane is removed from the solid-phase synthesizer that 2-chlorotrityl chloride resin is housed with nitrogen;
[0035] In the third step, 1 mmol of the compound 5 synthesized in the third step was dissolved in 2 mL of anhydrous dichloromethane, and 1 mL (ie 0.5 mmol) was taken, and 0.5 ...
Embodiment 3
[0045] Synthesis of the polypeptide Nap-FFY(p)-OMe shown in the following structural formula (II).
[0046]
[0047] The operation methods and parameters of other synthesis steps are the same as those in Example 2, except that the raw materials for polypeptide synthesis in steps 6-7 of the fourth step are replaced by Fmoc-phenylalanine and naphthaleneacetic acid.
[0048] Thus synthesized to obtain the polypeptide Nap-FFY(p)-OMe as shown in the above structural formula (II), the yield is 94%, 1 H NMR (400MHz, DMSO-d 6 )δ8.63 (d, J=7.324, 1H), 8.39 (d, J=8.518, 1H), 8.28 (d, J=8.216, 1H), 7.90-8.00 (m, 3H), 7.74 (s, 1H ), 7.62(m, 2H), 7.35-7.40(m, 3H), 7.25-7.40(m, 10H), 7.23-7.25(m, 2H), 4.6-4.8(m, 3H), 3.745(s, 3H ), 3.60-3.72(m, 3H), 3.000-3.120(m, 3H), 2.3-2.80(m, 2H). MS: calc.M + =737.25, obsvd.(M+Na) + =760.35.
[0049] The Nap-FFY(p)-OMe molecule of the polypeptide shown in the above structural formula (II) prepared in this example can be well dissolved in aqueo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com