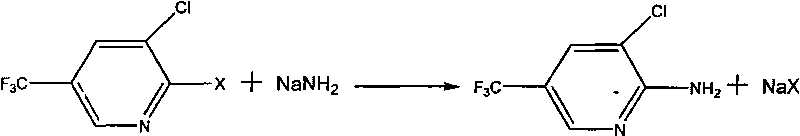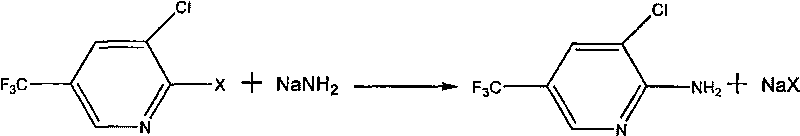Preparation method of 2-amino-3-chloro-5-(trifluoromethyl) pyridine
A technology of trifluoromethylpyridine and amino, applied in the field of fine chemistry, can solve the problems of harsh production conditions and low yield, and achieve the effects of mild reaction and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] The preparation of embodiment 1,2-amino-3-chloro-5-trifluoromethylpyridine
[0015] 4.7g 0.12mol NaNH 2 Pulverize and add to 60gDMF, under nitrogen protection, stir and heat to reflux, then add 21.6g 0.1mol 2,3-dichloro-5-trifluoromethylpyridine dropwise to the reaction solution, under nitrogen protection, stir and heat to reflux, and control raw materials After the reaction was complete, the reaction was terminated, filtered, the filtrate was precipitated under reduced pressure, washed with water, and dried to obtain 16.8 g of 2-amino-3-chloro-5-trifluoromethylpyridine with a purity of 98% and a yield of 83.7%.
Embodiment 2
[0016] The preparation of embodiment 2,2-amino-3-chloro-5-trifluoromethylpyridine
[0017] 4.7g 0.12mol NaNH 2 Add 1.5g of 0.025mol ethanolamine into 60g of xylene, nitrogen protection, stir and heat to reflux, then add dropwise 20.0g 0.1mol 2-fluoro-3-chloro-5-trifluoromethylpyridine to the reaction solution, nitrogen Protected, stirred and heated to reflux, the reaction of the central control raw material is complete, the reaction is terminated, filtered, the filtrate is precipitated under reduced pressure, washed with water, and dried to obtain 19.1g of 2-amino-3-chloro-5-trifluoromethylpyridine with a purity of 96% , yield 93.4%.
Embodiment 3
[0018] The preparation of embodiment 3,2-amino-3-chloro-5-trifluoromethylpyridine
[0019] 5.5g 0.14mol NaNH 2 Grinding and adding 6.7g 0.03mol methyl tributyl ammonium chloride to 60g decane, under nitrogen protection, stirred and heated to reflux, then added dropwise 20.0g 0.1mol 2-fluoro-3-chloro-5- Trifluoromethylpyridine, nitrogen protection, stirring and heating to reflux, the reaction of the central control raw material is complete, the reaction is completed, filtered, the filtrate is precipitated under reduced pressure, washed with water, and dried to obtain 2-amino-3-chloro-5-trifluoromethyl Pyridine 19.0g, purity 95%, yield 92.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com