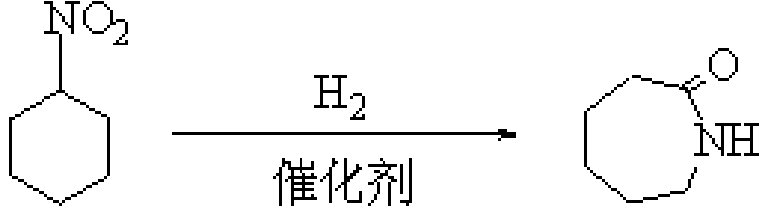
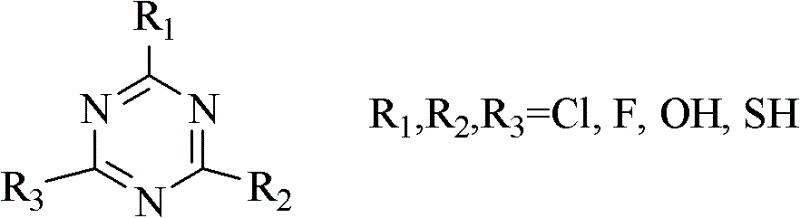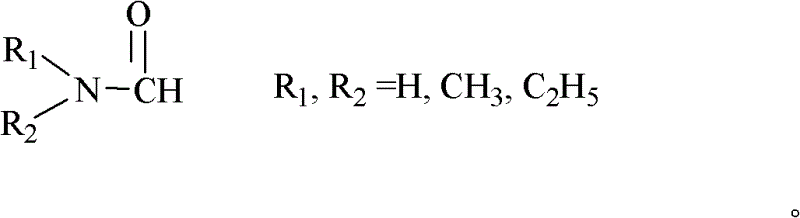Method for synthesizing caprolactam in one step by liquid phase hydrogenation of nitrocyclohexane
A nitrocyclohexane liquid and a technology for nitrocyclohexane are applied in the field of one-step synthesis of caprolactam by liquid-phase hydrogenation of nitrocyclohexane, can solve the problems of complicated method, a large amount of concentrated sulfuric acid, expensive separation of catalysts, etc. The effect of simplified process and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1.84 g of 2,4,6-trichloro-1,3,5-triazine was dissolved in 10 ml of N,N-dimethylformamide, and the mixture was stirred at room temperature for a period of time and then added to the reaction kettle, Then add 1.29 grams of nitrocyclohexane and 0.2 grams of 5% Pd / C to the kettle, connect the reaction kettle to the hydrogen cylinder, check the airtightness, feed hydrogen and evacuate, repeat five times, to exhaust other gases as much as possible , and then reacted for 6h in an oil bath at 80°C under a hydrogen pressure of 0.2MPa. The reacted mixture was filtered, and the filtrate was analyzed by gas chromatography and the internal standard method. It was calculated that the conversion rate of nitrocyclohexane was 63.87%, and the selectivity of caprolactam was 15.43%.
Embodiment 2
[0026] 1.84 g of 2,4,6-trichloro-1,3,5-triazine was dissolved in 10 ml of N,N-dimethylformamide, and the mixture was stirred at room temperature for a period of time and then added to the reaction kettle, Then add 1.29 grams of nitrocyclohexane and 0.2 grams of 5% Pd / C to the kettle, connect the reaction kettle to the hydrogen cylinder, check the airtightness, feed hydrogen and evacuate, repeat five times, to exhaust other gases as much as possible , and then reacted for 6h in an oil bath at 110°C under a hydrogen pressure of 0.35MPa. The reacted mixture was filtered, and the filtrate was analyzed by gas chromatography and the internal standard method. It was calculated that the conversion rate of nitrocyclohexane was 77.31%, and the selectivity of caprolactam was 7.12%.
Embodiment 3
[0028] 1.84 g of 2,4,6-trichloro-1,3,5-triazine was dissolved in 10 ml of N,N-dimethylformamide, and the mixture was stirred at room temperature for a period of time and then added to the reaction kettle, Then add 1.29 grams of nitrocyclohexane and 0.1 gram of 15% Ni / C to the kettle, connect the reaction kettle to the hydrogen cylinder, check the airtightness, pass in hydrogen and evacuate, repeat five times, to exhaust other gases as much as possible , and then reacted for 6h in an oil bath at 80°C under a hydrogen pressure of 0.2MPa. The reacted mixture was filtered, and the filtrate was analyzed by gas chromatography and the internal standard method. It was calculated that the conversion rate of nitrocyclohexane was 92.35%, and the selectivity of caprolactam was 9.73%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com